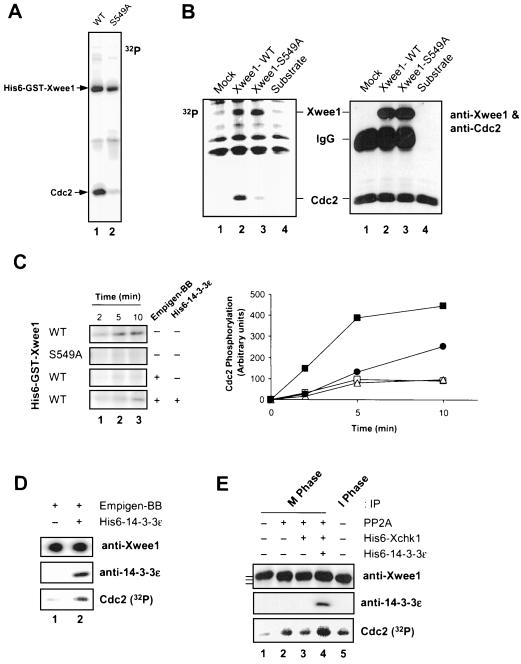
Figure 4.
Binding of 14-3-3 proteins to Xwee1 increases its kinase activity toward Cdc2. (A) Baculovirus-expressed wild-type (WT) (lane 1) and S549A (lane 2) His6-GST-Xwee1 were assayed for kinase activity toward a recombinant, kinase-inactive Cdc2–cyclin B complex (see MATERIALS AND METHODS). The kinase reactions were subjected to SDS-PAGE and autoradiography to detect incorporation of 32P into Cdc2. (B) Reticulocyte lysates containing no expressed Xwee1 (lane 1), Xwee1-WT (lane 2), or Xwee1-S549A (lane 3) were immunoprecipitated with anti-Xwee1 antibodies. The immunoprecipitates were incubated with a recombinant Cdc2–cyclin B complex (lanes 1–3) in the presence of [32P]ATP. Lane 4 depicts a control in which Cdc2–cyclin B was incubated without any anti-Xwee1 immunoprecipitate. The incubations were subjected to SDS-PAGE and analyzed by both autoradiography (left) and immunoblotting with a mixture of anti-Xwee1 and anti-Cdc2 antibodies (right). (C) Removal and rebinding of 14-3-3 reversibly affects the kinase activity of Xwee1. His6-GST-Xwee1-WT and His6-GST-Xwee1-S549A (as indicated) were incubated in the absence (−) or presence (+) of 0.4% Empigen-BB (a detergent that removes 14-3-3 proteins from Xwee1). After washing, the samples were incubated in the absence (−) or presence (+) of His6–14-3-3ε. Finally, the samples were mixed with a recombinant Cdc2–cyclin B complex. The kinase reactions were terminated after 2 min (lane 1), 5 min (lane 2), or 10 min (lane 3) and processed for SDS-PAGE and autoradiography to detect phosphorylation of Cdc2 (left). The right panel depicts quantitation of the data. Symbols: wild-type Xwee1 (▪); S549A mutant Xwee1 (□); Empigen-treated, wild-type Xwee1 that was incubated further in the absence (▵) or presence of His6–14-3-3ε (●). (D) Endogenous Xwee1 from interphase Xenopus egg extracts was immunoprecipitated with anti-Xwee1 antibodies, treated with Empigen-BB, washed, incubated in the absence (lane 1) or presence (lane 2) of His6–14-3-3ε, and assayed for Cdc2-specific kinase activity. The samples were subjected to SDS-PAGE and processed for immunoblotting with anti-Xwee1 antibodies (top), immunoblotting with anti-14-3-3ε antibodies (middle), or autoradiography to detect phosphorylation of Cdc2 (bottom). (E) Respective contributions of M-phase phosphorylation and Xchk1/14-3-3 to the regulation of Xwee1 during the cell cycle. Endogenous Xwee1 was immunoprecipitated with anti-Xwee1 antibodies from M-phase (lanes 1–4) or interphase (lane 5) egg extracts. The immunoprecipitates were treated in the presence (lanes 2–4) or absence (lanes 1 and 5) of PP2A. Subsequently, the samples were incubated with (lanes 3 and 4) or without (lanes 1, 2, and 5) His6-Xchk1. Next, His6–14-3-3ε (lane 4) was added to one incubation. Finally, a recombinant Cdc2–cyclin B complex and [32P]ATP were added. The kinase reaction samples were subjected to SDS-PAGE and processed for immunoblotting with anti-Xwee1 (top) or anti-14-3-3ε (middle) antibodies or for autoradiography to detect phosphorylation of Cdc2 (bottom). Kinase activity toward Cdc2 was quantitated and normalized to lane 1: lane 2 (3.6-fold), lane 3 (3.4-fold), lane 4 (5.5 fold), and lane 5 (3.5-fold). The bars to the left of top panel denote the various forms of Xwee1 with different electrophoretic mobilities.