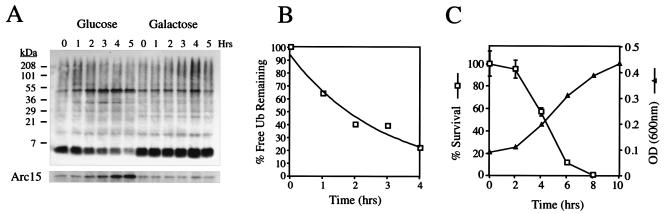
FIG. 6.
Determination of the half-life of ubiquitin. (A) SUB328 cells expressing a single galactose-inducible ubiquitin gene were grown in galactose in exponential phase. At time zero, cells were either transferred to glucose-containing media or maintained on galactose, and aliquots were taken at the indicated time points. For cells growing on glucose, an equal culture volume was taken at each time point to prevent dilution of the total ubiquitin pool by cell division; for cells growing on galactose, an equivalent number of cells were taken. Cells were analyzed by immunoblotting for ubiquitin (upper panel), followed by enhanced chemiluminescence. Loads were normalized by using an antibody to Arc15 as described previously (lower panel). (B) The immunoblot from panel A was stripped and reprobed with the same antiubiquitin antibody, followed by 125I-labeled protein A, and quantitated on a PhosphorImager with NIH Image. There was a lag time of approximately 1 h in ubiquitin (Ub) shutoff by glucose, and therefore the half-life was determined from 1 to 5 h postshift. (C) Cultures of SUB328 growing in glucose or galactose in exponential phase (as in panel A) were sampled at the indicated time point, and an equivalent amount of OD600 units were plated on YPRafGal plates. Colonies were counted 2 days later. The survival curve is expressed as the percent plating efficiency of the glucose-grown culture divided by that of the galactose culture (□). Error bars represent the standard deviation from an experiment carried out in triplicate. Cultures of SUB328 shifted to glucose media were normalized by OD and allowed to grow at 30°C in the exponential phase. ODs were determined at the indicated time points (▵).