Table 4.
Initial Rates of Aryl–CF3 Bond-Forming Reductive Elimination from Complexes 4-6
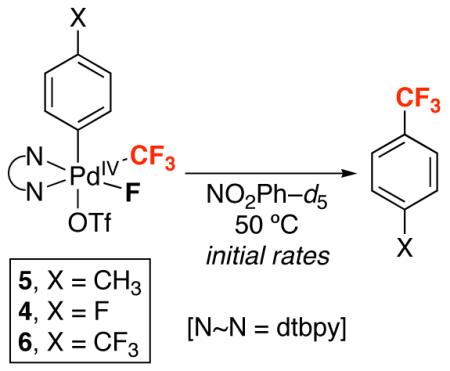
| Entry | X | Yield Aryl–CF3 | Initial rate (M s−1) × 105 |
|---|---|---|---|
| 1 | CH3 | 93% | 45.8 |
| 2 | F | 77% | 2.21 |
| 3 | CF3 | 65% a | 1.43 |
Reactions were run in duplicate and yields were determined by 19F NMR spectroscopy.
a1,4(bistrifluoromethyl)biphenyl formed in 21% yield.
