Table 7.
Synthesis and Reactivity of (tmeda)Pd(Aryl)(CF3) Complexes
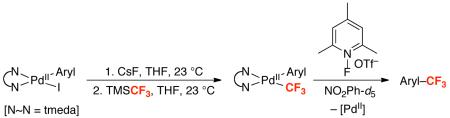
| Entry | Compound | Aryl | Yield Aryl–CF3 (80 °C) |
Yield Aryl–CF3 (23 °C) |
|---|---|---|---|---|
| 1 | 11a | p-FC6H4 | 81% | 78% |
| 2 | 11b | p-CF3C6H4 | 76%a | 52%a |
| 3 | 11c | p-CNC6H4 | 60% | 22% |
| 4 | 11d | p-MeOC6H4 | 92% | 95% |
| 5 | 11e | C6H5 | 94% | 88% |
| 6 | 11f | p-MeC6H4 | 90% | 83% |
| 7 | 11g | m-MeC6H4 | 95% | 95% |
| 8 | 11h | o-MeC6H4 | 85% | 88% |
| 9 | 11i | o-MeOC6H4 | 90% | 99% |
These reactions were conducted at 80 °C for 3 h and at 23 °C for 1 h. Reactions were run in duplicate and all of the starting material was consumed. Yields were determined by 19F NMR spectroscopy.
At both temperatures, trifluorotoluene was formed in 13% yield.
