FIG. 1. .
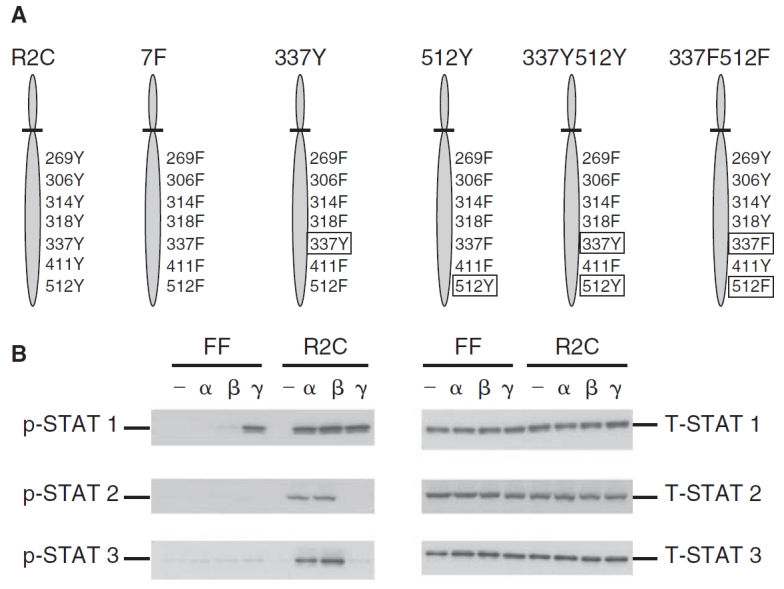
Phosphorylation of STAT1, STAT2, and STAT3 in the mutant IFNAR-2c (FF) and wild-type IFNAR-2c (R2C) cells. (A) A schematic representation of the tyrosine residues in the cytoplasmic domain of IFNAR-2c expressed in U5A cells. U5A cells expressing the wild-type IFNAR-2c with all 7 intact cytoplasmic tyrosine residues at 269, 306, 314, 318, 337, 411, and 512 (R2C); mutant IFNAR-2c in which all the above 7 tyrosines have been replaced with phenylalanine (7F); mutant IFNAR-2C with a single tyrosine at 337 (337Y); mutant IFNAR-2c with a single tyrosine at 512 (512Y); mutant IFNAR-2c with 2 tyrosines at 337 and 512 (337Y512Y) and mutant IFNAR-2c with 2 phenylalanines at 337 and 512 (337F512F) are shown. (B) FF and R2C cells were left untreated (—), or treated with interferon (IFN)-α2 (α) or IFN-β (β) (5,000 U/mL) or IFN-γ (γ) (1,000 U/mL) for 15 min and whole cell extracts prepared. Cell lysates were resolved by SDS-PAGE, blotted onto PVDF membranes, and subjected to immunoblotting using anti-phospho-STAT1, STAT2, or STAT3 (left set of 3 panels). Blots were stripped and re-probed with anti-STAT1, STAT2, or STAT3 (right set of panels) demonstrating that STAT1, STAT2, and STAT3 proteins were expressed at equivalent levels in both cell lines.
