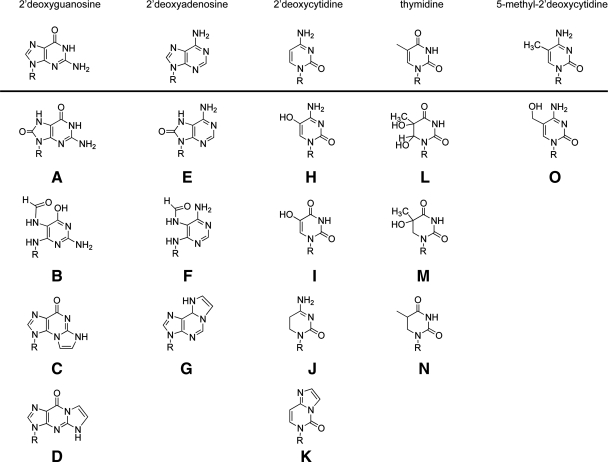
FIG. 1.
Oxidative damage of 2′-deoxynucleotides. Structures of 2′-deoxyguanosine and the oxidatively modified DNA lesions 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) (A); 2,6-diamino-4-hydroxy-5-formamidopyrimidine (FapydG) (B); the LPO products N2,3-etheno-2′-deoxyguanosine (C); and 1,N2-etheno-2′-deoxyguanosine (D) (15, 19, 74). Structures of 2′-deoxyadenosine and the oxidatively modified DNA lesions 8-oxo-7,8-dihydro-2′-deoxyadenosine (8-oxodA) (E); 4,6-diamino-5-formamidopyrimidine (FapydA) (F); and the LPO product 1,N6-etheno 2′-deoxyadenosine (G) (15, 19, 74). Structures of 2′-deoxycytidine and the oxidatively modified DNA lesions 5-hydroxy-2′-deoxycytidine (OH5dC) (H); 5-hydroxy-2′-deoxyuridine (OH5dU) (I); 5,6-dihydro-2′-deoxyuridine (dHU) (J); and the LPO product 3,N4-ethenocytidine (K) (15, 19, 74). Structures of thymidine and the oxidatively modified DNA lesions thymine glycol (Tg) (L); 5-hydroxy-5,6-dihydrothymine (Th5) (M); and 5,6-dihydrothymine (dHT) (N) (15, 19). Structures of 5-methyl-2′-deoxycytidine and the oxidatively modified DNA lesion 5-(hydroxymethyl)-2′-deoxycytidine (hmdC) (O) (15). Where indicated, the R-group is 2′-deoxyribose.