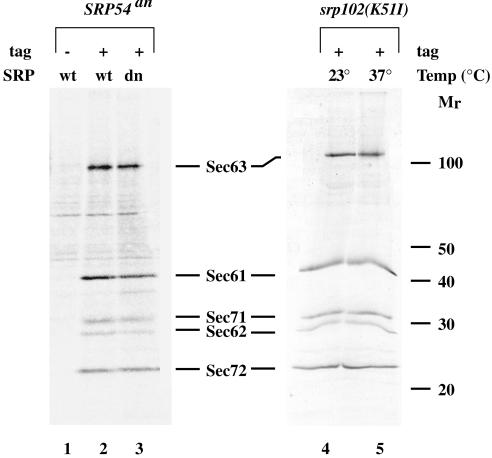
Figure 3.
The translocon in adapted cells is identical to that of wild-type cells. Sec63 complex proteins were purified from the following strains: SMY212 (lane 1, no tag); SMY268 bearing pSM131 (lane2, Sec64prA); SMY268 bearing pSM110 (lane 3, Sec63prA); srp102::URA3, SMY266 (lane 4 and 5, Sec63prA) as described in MATERIALS AND METHODS. Cells were grown in raffinose-containing medium to midlog phase and switched to growth in galactose-containing medium lacking methionine. Cultures were maintained in log phase in galactose-containing medium for 24 h (lanes 1–3). Alternatively, cultures were grown to midlog phase in YPD at 23°C (lane 4 and 5) and switched to 37°C for 12 h (lane 5). Cells were steady-state labeled with [35S]methionine for 45 min to 1 h at 30°C (lanes 1–3), 23°C (lane 4), or 37°C (lane 5), and membranes were isolated. Digitonin extracts of membranes were incubated with IgG Sepharose for 3 h at 4°C with rotation. The IgG Sepharose beads were washed and protein complexes were eluted with 100 mM glycine pH 2.0. The eluate was concentrated by TCA precipitation and analyzed by SDS-PAGE. The major protein components of the translocon are indicated.