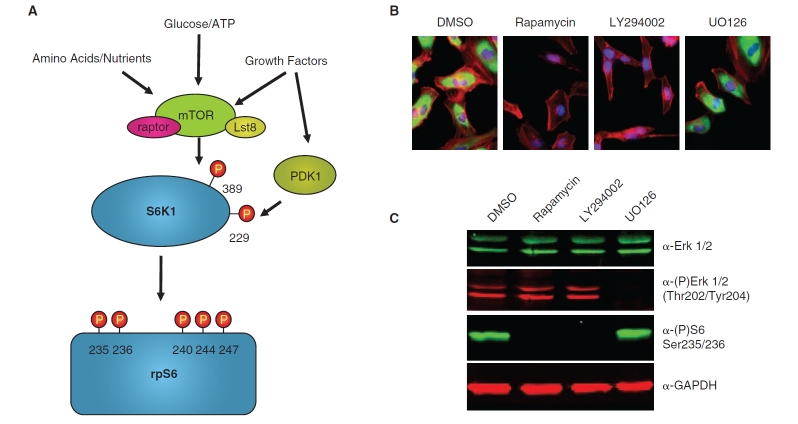
Fig. 1. .
rpS6 phosphorylation as an endpoint for mTORC1 signaling. (A) A schematic of the signaling events leading to rpS6 phosphorylation. mTORC1 kinase activity is regulated by multiple upstream signals, including growth factors, cellular energy status, and amino acid availability, and phosphorylates S6K1 on T389 in the hydrophobic motif (numbering based on p70 S6K1). PDK1 is regulated by growth factor signaling and phosphorylates S6K1 on T229 in the catalytic domain. Once fully activated by these 2 phosphorylation events, S6K1 then phosphorylates rpS6 on multiple sites. The antibody used for the In Cell Western assay described here recognizes rpS6 phosphorylated on both Ser235 and Ser236. (B) HeLa cells were incubated with the indicated compounds for 3 h, then fixed and processed for immunofluorescence. rpS6-phosphorylation was visualized by staining with the anti-phospho-S6 S235/S236 antibody and an Alexa-488 anti-rabbit secondary antibody (green), actin filaments were stained with Alexa-568-conjugated phalloidin (red), and nuclei were stained with Hoechst (blue). rpS6 phosphorylation is lost following treatment with the pathway-specific inhibitors, rapamycin and LY294002, but not with the MEK inhibitor U0126 or the DMSO vehicle control. (C) HeLa cells were incubated with the indicated compounds for 3 h, then lysed and processed for western blot analysis and probed with the indicated primary antibodies and either the goat anti-rabbit IRDye-800CW or goat anti-mouse IR-Dye700 secondary antibodies. The blots were visualized on the Odyssey infrared imager (LI-COR Biosciences). A reduction in rpS6-phosphorylation was observed upon treatment with the pathway-specific inhibitors, rapamycin and LY294002, but not with the MEK inhibitor U0126 or the DMSO vehicle control. The ability of U0126 to inhibit MEK activity is confirmed by the loss in Erk phosphorylation. GAPDH is shown as a loading control.