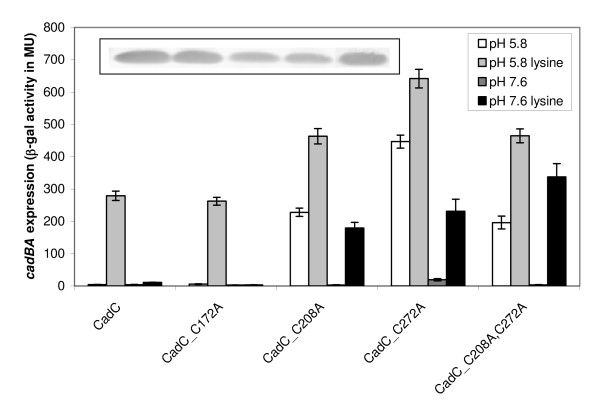
Figure 1.
Influence of cysteine replacements in CadC on cadBA expression. Reporter gene assays were performed with E. coli EP314 (cadC::Tn10; cadA'::lacZ fusion) which was complemented with plasmid-encoded cadC or the indicated cadC derivatives. Cells were cultivated under microaerobic conditions in minimal medium at pH 5.8 or pH 7.6 in the presence or absence of 10 mM lysine at 37°C to mid-logarithmic growth phase, and harvested by centrifugation. The activity of the reporter enzyme β-galactosidase was determined [43] and served as a measurement for cadBA expression. Error bars indicate standard deviations of the mean for at least three independent experiments. To analyze production and membrane integration of the CadC derivatives, Western blot analysis of membrane fractions from E. coli BL21(DE3)pLysS transformed with plasmids encoding either wild-type or CadC derivatives was performed (inset). Each lane contains 25 μg of membrane protein (CadC derivatives are in the same order as in the graph). CadC was detected by a monoclonal mouse antibody against the His-Tag and an alkaline phosphatase coupled anti-mouse antibody.