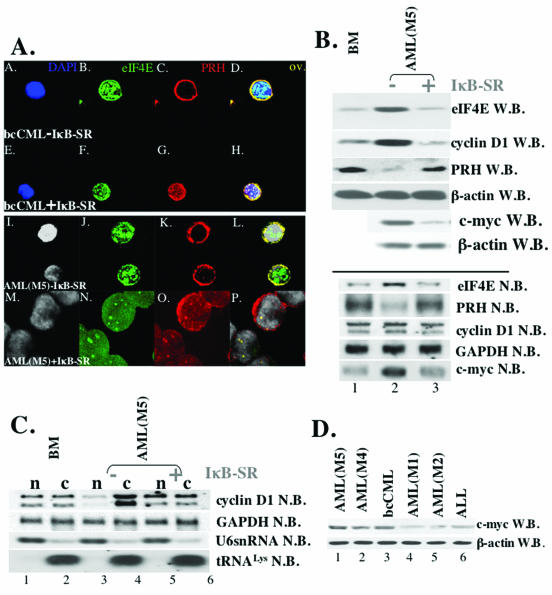
FIG. 3.
Expression of IκB-SR in CD34+ cells, derived from M5 AML and bcCML patients, correlates with the downregulation of the c-myc expression and the restoration of the expression and subcellular distribution of eIF4E and PRH proteins. (A) Cells were stained with anti-eIF4E Ab, followed by Texas red-conjugated anti-mouse IgG Ab (shown in green) and anti-PRH Ab, followed by Cy5-conjugated anti-rabbit IgG Ab (red). Nuclei were stained with DAPI (blue in panels A and E; gray in panels I and M). The PRH-eIF4E overlay (ov.) is shown in yellow. The objective was a 100× lens with a further magnification of two (A to L)- or three (G to R)-fold. (B) Western (W.B.) and Northern (N.B.) blot analysis of CD34+ cells derived from the healthy individuals (BM) and M5 AML patients [AML(M5)-IκB]. Blots were probed as indicated. (C) IκB-SR expression correlates with the restoration of cyclin D1 mRNA transport. Northern blot analysis of RNA isolated from nuclear (n) and cytoplasmic (c) fractions of CD34+ cells derived from healthy individuals (BM) and M5 AML patients. In contrast to Ad-GFP-transduced cells (−IκB-SR), CD34+ M5 AML cells that express IκB-SR (+IκB-SR) showed the same subcellular distribution of cyclin D1 mRNA (lanes 5 and 6) as CD34+ BM cells. tRNALys and U6snRNA were used as markers for cytoplasmic and nuclear fractions, respectively. −IκB-SR, cells transduced with Ad-GFP; +IκB-SR, cells transduced with Ad-GFP-IκB-SR. (D) Western blot analysis reveals upregulated levels of c-myc in M4 AML, M5 AML, and bcCML specimens (c-myc W.B.). β-Actin is shown as a control for protein loading.