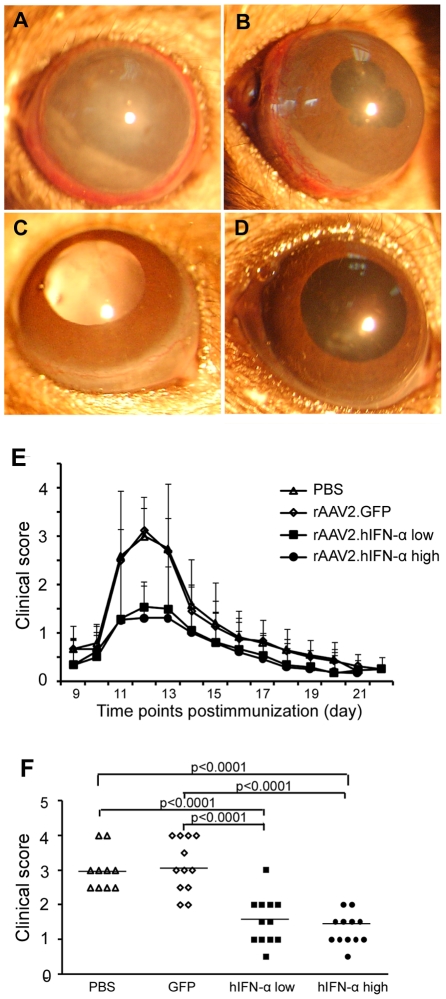
Figure 4. Clinical evaluation of EAU activity.
Two doses of AAV2.hIFN-α were subretinally injected into the eye respectively, PBS and AAV2.GFP were used as controls. Three weeks after injection, EAU was induced by immunization with IRBP161–180 and ocular inflammation was examined by slit lamp microscopy. Images show significantly severe inflammation in the PBS (A) and AAV2.GFP injected eyes (B) as compared to the AAV2.hIFN-α treated eyes (C, D). Kinetics of EAU (E) reveals that subretinal injection of both doses of AAV2.hIFN-α persistently attenuated ocular inflammation of EAU as compared with PBS and AAV2.GFP. The significant difference was observed consecutively on day 11 to 14 after immunization (P<0.05). Data are presented as mean ± standard deviation. Clinical score on day 12 after immunization (F) shows that the PBS injected eyes had a score of 3 (±0.58) and the AAV2.GFP injected eyes reached a mean clinical score of 3.13 (±0.77), the score of AAV2.hIFN-α treated eyes was 1.54 (±0.69, p<0.0001, Mann-Whitney U test) in the lower dose treated group and 1.292 (±0.45, p<0.0001) in the higher dose group. Each point represents an individual eye. The average scores of each group are denoted by the horizontal bars.