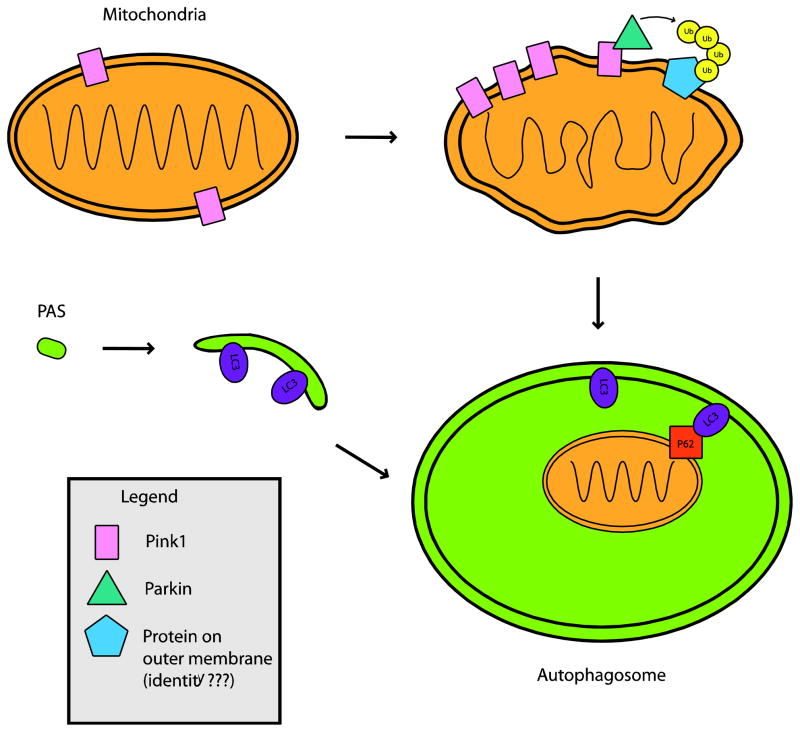
Figure 1. PINK1 and Parkin interact to recruit mitochondria to autophagosomes, and thereby promote mitophagy.
PINK1 is present on mitochondria normally, but upon mitochondrial depolarization, PINK1 rapidly accumulates on mitochondria. This is followed by recruitment of Parkin, and the subsequent poly-ubiquitination of an unknown target protein by Parkin. Poly-ubiquitinated mitochondria are shuttled to developing autophagosomes through an interaction between the poly-ubiquitin chain, p62, and LC3 on the autophagosome membrane. Once inside an autophagosome, depolarized mitochondria can be degraded by lysosomes, when autophagosome-lysosome fusion occurs (not shown). In Parkinson’s disease caused by recessive PINK1 or Parkin mutations, depolarized mitochondria are no longer efficiently degraded by this pathway, contributing to the hallmark mitochondrial dysfunction observed in this disorder.
PAS = Pre-autophagosomal structure