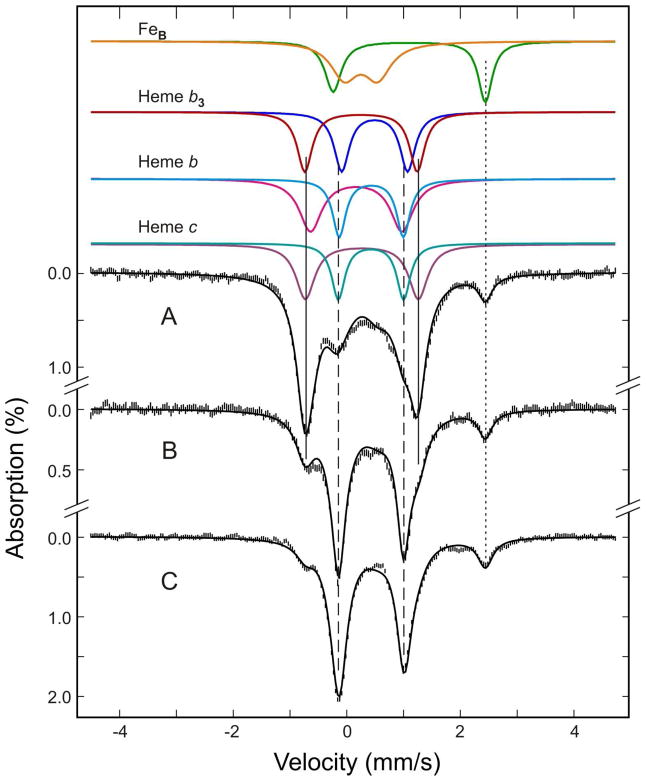
Figure 5.
Mössbauer spectra of the as-purified (A) ascorbate-reduced (B) and dithionite-reduced (C) Ps. nautica cNOR (805 μM in 100 mM KPB, pH 7, 0.02% DDM, 0.01% PE). The spectra (vertical bars) were collected at 180 K in a weak magnetic field of 50 mT. The color lines displayed above the data are the de-convoluded spectra for the for Fe cofactors in the enzyme: FeBIII, orange; FeBII, green; FeIII-heme b3, red; FeII-heme b3, blue; FeIII-heme b, magenta; FeII-heme b, cyan; FeIII-heme c, purple; FeII-heme c, blue-green. The back solid lines overlaid with the experimental spectra are composite spectra simulated with the parameters and percent absorptions listed in Tables 1. The dotted vertical line indicates the position of the high-energy line of the FeBII doublet. The two solid vertical lines and the two dashed vertical lines, respectively, mark the general line positions of the doublets arising from the low-spin FeII-heme and FeIII-heme cofactors.