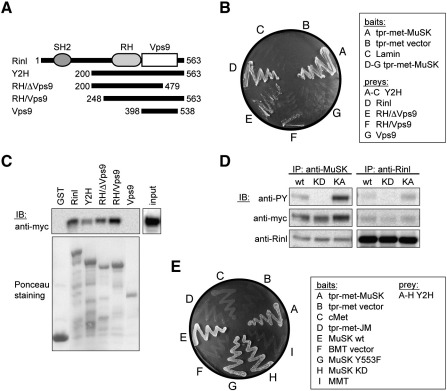
Fig. 1.
Rinl is a specific binding partner of MuSK. (A) A scheme of Rinl and corresponding truncation mutants. (B) Yeast was transformed with bait and prey constructs as indicated. Interaction was assayed by growth of yeast clones on –HIS. (C) Rinl GST-deletion constructs were purified from bacteria and used for a pulldown of MuSK-myc expressed in HEK 293T cells. Proteins were detected by immunoblotting (IB) using anti-myc antibodies. GST protein input was assayed by Ponceau staining. 5% of the total MuSK-myc input is shown in the right panel. (D) MuSK-myc and Rinl were expressed in HEK 293T cells and co-immunoprecipitated either with anti-MuSK antibodies or anti-Rinl antibodies. Immunoprecipitated proteins were assayed by immunoblotting using anti-myc and anti-Rinl antibodies, respectively. Phosphorylated MuSK was detected with anti-phosphotyrosine (PY). wt, wild-type; KD, kinase-dead; KA, kinase-active. IP, immunoprecipitation. (E) Yeast was transformed with the indicated MuSK bait constructs and the Rinl (Y2H) prey construct. Interaction was followed by growth of yeast clones on –HIS.