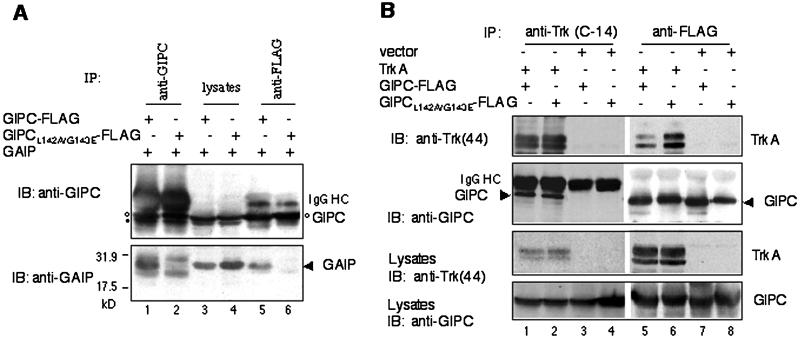
Figure 7.
TrkA and GAIP bind to different sites in the PDZ domain of GIPC. (A) Lack of interaction between GIPC(L142A/G143E) and GAIP. C-terminal FLAG-tagged GIPC (lanes 1, 3, and 5) or GIPC(L142A/G143E) mutant (lanes 2, 4 and 6) were coexpressed with GAIP in HEK293 cells. GIPC was immunoprecipitated (IP) from cell lysates with anti-GIPC (lanes 1 and 2) or anti-FLAG (M2) antibody (lanes 5 and 6), and the precipitates were immunoblotted (IB) with anti-GIPC (top) and anti-GAIP (bottom). GAIP coprecipitated with wild-type GIPC (lanes 1 and 5) but not with the GIPC(L142A/G143E) mutant (lanes 2 and 6). Protein expression levels (lanes 3 and 4) were comparable. The double bands at ∼40 kDa (top, lanes 1–4) represent endogenous GIPC (•) and overexpressed GIPC-FLAG (○). IgG HC, IgG heavy chain. The two bands immediately above and below GAIP (lanes 1 and 2) are IgG light chains. (B) GIPC(L142A/G143E) binds TrkA. C-terminal FLAG-tagged GIPC or GIPC(L142A/G143E) were coexpressed with TrkA (lanes 1, 2, 5, and 6) or empty vector (lanes 3, 4, 7, and 8) in HEK293 cells. Lysates were immunoprecipitated with anti-TrkA (C-14) (lanes 1–4) or anti-FLAG (lanes 5–8) IgG followed by immunoblotting with anti-TrkA (44) (top panel) or anti-GIPC (second panel). Both wild-type GIPC (lane 1) and the GIPC mutant (lane 2) coprecipitate with TrkA in cells cotransfected with TrkA. Similarly, TrkA coprecipitates with both wild-type GIPC-FLAG (lane 5) and mutant GIPC-FLAG (lane 6). No GIPC (lanes 3 and 7) or GIPC(L142A/G143E) (lanes 4 and 8) was precipitated from samples cotransfected with empty vector. Protein expression levels of TrkA (third panel) and GIPC (bottom panel) are shown.