Abstract
Epidemiological studies have shown that adolescent smoking is associated with health risk behaviors, including high-risk sexual activity and illicit drug use. Using rat as an animal model, we evaluated the behavioral and biochemical effects of a 4-day, low-dose nicotine pretreatment (60 μg/kg; intravenous) during adolescence and adulthood. Nicotine pretreatment significantly increased initial acquisition of cocaine self-administration, quinpirole-induced locomotor activity, and penile erection in adolescent rats, aged postnatal day (P)32. These effects were long lasting, remaining evident 10 days after the last nicotine treatment, and were observed when nicotine pretreatment was administered during early adolescence (P28–31), but not late adolescence (P38–41) or adulthood (P86–89). Neurochemical analyses of c-fos mRNA expression, and of monoamine transmitter and transporter levels, showed that forebrain limbic systems are continuing to develop during early adolescence, and that this maturation is critically altered by brief nicotine exposure. Nicotine selectively increased c-fos mRNA expression in the nucleus accumbens shell and basolateral amygdala in adolescent, but not adult animals, and altered serotonin markers in these regions as well as the prefrontal cortex. Nicotine enhancement of cocaine self-administration and quinpirole-induced locomotor activity was blocked by co-administration of WAY 100 635 (N-{2-[4-(2-methoxyphenyl)-1-piperazinyl] ethyl}-N-(2-pyridinyl)cyclohexanecarboxamide), a selective serotonin 1A (5-HT1A) receptor antagonist. Early adolescent pretreatment with the mixed autoreceptor/heteroceptor 5-HT1A receptor agonist, 8-OH-DPAT, but not the autoreceptor-selective agonist, S-15535, also enhanced quinpirole-induced locomotor activation. Nicotine enhancement of quinpirole-induced penile erection was not blocked by WAY 100 635 nor mimicked by 8-OH-DPAT. These findings indicate that early adolescent nicotine exposure uniquely alters limbic function by both 5-HT1A and non-5-HT1A receptor mechanisms.
Keywords: dopamine, locomotion, penile erection, self-administration, serotonin
INTRODUCTION
Smoking and neuropsychiatric disease often co-occur, with the onset of both disorders emerging during adolescence (Covey et al, 2008; Gehricke et al, 2007; Weiser et al, 2004). The adolescent brain undergoes dynamic maturation of limbic circuitry and of efferent dopamine (DA) and serotonin (5-HT) neurotransmitter systems, which are common components that underlie the rewarding aspects of smoking, as well as neuropsychiatric pathologies. It has been proposed that exposure to nicotine during this critical period may trigger long-term changes in neural signaling, and increase likelihood for illicit drug use, health risk behaviors, and mood disorders later in life (Bronisch et al, 2008; Isensee et al, 2003; John et al, 2004). Indeed, adolescent smokers have disproportionately high rates of co-morbid high-risk sexual behavior (Biglan et al, 1990; Lam et al, 2001) and psychiatric disorders, including attention deficit hyperactivity disorder (ADHD), mood disorders (Gehricke et al, 2007), and substance abuse (Kandel et al, 1992). Longitudinal clinical studies have also suggested a ‘gateway' effect of early adolescent smoking on later substance abuse (Kandel et al, 1992; Lewinsohn et al, 1999; Brook et al, 2007; Wagner and Anthony, 2002), a finding that is more significant in patients with ADHD (Biederman et al, 2006; Wilens et al, 2008). Despite clear clinical implications, the neurobiological mechanisms that underlie the associations between adolescent tobacco use and co-morbid psychopathologies have not been defined.
Adolescence is a critical transitional period of development that occurs between childhood and adulthood. This generally falls between 12 and 18 years in human beings, and postnatal day (P) 28–42 in rodents (Spear, 2000). Both human and rodent adolescents experience similar social and psychological changes, with the emergence of novelty seeking, risk taking, and peer association behaviors, that are thought to facilitate the transition to independence and autonomy (Spear, 2000).
Animal studies have confirmed that adolescents are vulnerable to persisting behavioral and neurochemical changes following brief, low-dose exposure to nicotine (Adriani et al, 2004; Abreu-Villaç et al, 2010; McQuown et al, 2007; Abreu-Villaca et al, 2003). We have previously shown that 4-day pretreatment during early adolescence with a low dose of nicotine (60 μg/kg, intravenous), equivalent to that found in 1–2 cigarettes per day, increases sensitivity to the initial reinforcing effects of cocaine (McQuown et al, 2007), and enhances induction and expression of cocaine locomotor sensitization (McQuown et al, 2009). Such nicotine-induced changes are unique to adolescents and do not occur in adults.
Nicotinic acetylcholine receptors (nAChRs) are known to modulate maturation of neural systems during critical developmental periods in which they are sensitive to environmental influences (Dwyer et al, 2009). Early adolescence is a sensitive period in which nAChR activation may have unique effects on the actively maturing limbic system. In particular, the prefrontal cortex (PFC)–basolateral amygdala (BLA)–nucleus accumbens (NAc) triadic circuit, responsible for reward and motivated behavior, is dynamically developing during this period, as are its monoamine afferents (Ernst et al, 2006; Ernst and Fudge, 2009). Mesolimbic DA systems, which are integral to cocaine self-administration and locomotor sensitization (Thomas et al, 2008), undergo major reorganization during adolescence (Tarazi et al, 1999; Cao et al, 2007b; Benoit-Marand and O'Donnell, 2008; Brenhouse et al, 2008; Andersen et al, 2000) and exhibit unique sensitivity to nicotine (Azam et al, 2007). Less is known about the adolescent maturation of 5-HT systems, which have a significant role in cocaine self-administration (Rocha et al, 1997; Rodd et al, 2005; Fletcher et al, 2002) and locomotor sensitization (Tassin, 2008). Although generally believed to develop early (Murrin et al, 2007), 5-HT neurons have been shown to undergo synaptic changes during early adolescence (Dinopoulos et al, 1997; Dori et al, 1998). Nicotine has also been shown to induce a striking increase in extracellular 5-HT levels in the NAc of adolescent rats (Shearman et al, 2008), and the 5-HT1A-receptor (5-HT1A-R) is uniquely modulated by chronic nicotine exposure in adolescence (Slotkin et al, 2007). These findings suggest that 5-HT may also contribute to the behavioral alterations induced by brief nicotine exposure in adolescence. The aim of this study was therefore to evaluate the role of DA and 5-HT afferents to the PFC–NAc–BLA triadic circuit in mediating the unique effects of adolescent nicotine exposure on subsequent responses to cocaine and other abused drugs.
MATERIALS AND METHODS
Animals
Male Sprague–Dawley rats (Charles River) were obtained as juveniles aged P17 with dams, or as adults aged P74. Young rats, after weaning at P21, and adults were group-housed in an AALAC-accredited temperature (21°C) and humidity (50%)-controlled vivarium, on a 12-h light–dark cycle with food and water available ad libitum. Only one animal per litter per experimental group was used. All experimental procedures were in compliance with the NIH guidelines and were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine, CA.
Surgical Procedure
Before pretreatment, rats were surgically prepared with a chronic catheter implanted into the right external jugular vein, as described by Belluzzi et al (2005). Animals were anesthetized with Equithesin (0.3 ml/100 g). A catheter was passed subcutaneously and implanted in the jugular vein. The cannula was flushed daily with sterile heparinized saline solution (600 U heparin per 30 ml saline) to maintain patency. All animals were given 3 days to recover before beginning experiments. After experiment, catheters were tested for patency with propofol, a rapid anesthetic. Data were discarded from animals not showing immediate anesthesia.
Drugs
Cocaine and d-methamphetamine hydrochloride were provided by the National Institute of Drug Abuse. Nicotine, MLA (1α,4(S),6β,14α,16β-20-ethyl-1,6,14,16-tetramethoxy-4-[[[2-(3-methyl-2,5-dioxo-1-pyrrolidinyl)benzoyl]oxy]methyl]aconitane-7,8-diol citrate salt), DHβE (3β-1,6-didehydro-14,17-dihydro-3-methoxy-16(15H)-oxaerythrinan-15-one hydrobromide), S-15535 (1-(2,3-dihydro-1,4-benzodioxin-5-yl)-4-(2,3-dihydro-1h-inden-2-yl)-piperazine), and WAY 100 635 (N-{2-[4-(2-methoxyphenyl)-1-piperazinyl] ethyl}-N-(2-pyridinyl)cyclohexanecarboxamide) were purchased from Sigma (St Louis, MO), (−)-quinpirole hydrochloride ((4aR-trans)-4,4a,5,6,7,8,8a,9-octahydro-5-propyl-1H-pyr azolo[3,4-g]quinoline hydrochloride) from Tocris Biosciences, and propofol from Abbot Laboratories (Chicago, IL). S-15535 was dissolved in sterile saline, plus a few drops of lactic acid, and pH was adjusted to as close to neutrality as possible (>5.0). The remaining drugs were dissolved in sterile saline and all drugs were filtered through sterile filters (Millipore Millex Sterile Filters, 0.22 pore, 3.3 mm diameter). Nicotine concentrations (pH 7.4) are expressed as nicotine base.
4-Day Pretreatment
The same design was used as described in McQuown et al (2007, 2009). Two intravenous injections of nicotine (2 × 30 μg/kg/0.1 ml), or saline, spaced 1 min apart, were administered daily for 4 consecutive days during early adolescence (P28–31), late adolescence (P37–40), or adulthood (P86–89). The daily nicotine dose used yields peak serum levels of approximately 30 ng/ml in both adolescents and adults (Cao et al, 2007a), which is well within the range of the average smoker. Intravenous injections of S-15535 (2 × 15 or 2 × 150 μg/kg) and 8-OH-DPAT (2 × 5 μg/kg) were administered during early adolescence (P28–31). In vivo studies show that administration of S-15535 intravenously at these doses significantly reduced dorsal raphe firing compared with baseline (Millan et al, 1993). Intravenous injection at this dose of 8-OH-DPAT was found to cause minimal aversive side effects (tail flicks, hypothermia, and lip retractions) (Bagdy and To, 1997). The selective 5-HT1A-R antagonist, WAY 100 635 (0, 40, or 400 μg/kg), was co-administered intravenously with nicotine or saline. These doses were selected because they do not modify spontaneous firing of 5-HT neurons or basal locomotor activity (Carey et al, 2001; Haddjeri et al, 2004). The selective nAChR antagonists, DHβE (2 mg/kg) and MLA (1 mg/kg), were administered intravenously 5 min before daily nicotine pretreatment. These doses are in the range of those previously reported to be effective in blocking each specific nAChR subtype in the brain (Solinas et al, 2007; Uchida et al, 2009).
Intravenous Self-administration
Animals were placed into an operant chamber equipped with two nose poke holes, and were tested in a single 2 h session on a fixed ratio 1 schedule to deliver a 0.02 ml intravenous infusion dose of cocaine (0.5 mg/kg), methamphetamine (0.02 mg/kg), or ethanol (1 mg/kg). During each 1.1 s infusion, a signal light above the reinforced hole illuminated, after which the house light shut off for a 3-s (ethanol) or 20-s (cocaine and methamphetamine) time-out period. To control for nonspecific activating effects of drugs, activity on a second (inactive) hole, whose activation had no programmed consequences, was recorded. A maximum of 100 infusions was allowed.
DA-Mediated Behaviors
Locomotion was measured using five identical open field activity chambers (43.2 × 43.2 × 30.5 cm3) connected to a common interface and computer (MED Associates, St Albans, VT). Horizontal movement was recorded by 16 photobeams per side evenly spaced along each wall of two adjacent sides. Before testing, animals were placed in the locomotor apparatus for a 30 min habituation period, after which they received an intraperitoneal injection of saline or quinpirole (0–1.6 mg/kg, intraperitoneal) and monitored for locomotion and stereotypic behaviors. Locomotor activity was recorded automatically at 5-min intervals during the subsequent 30-min test period. An observer, blind to the treatment groups, also observed each animal for 10 s of every minute and assigned a stereotypy score based on a modified scale of LaHoste and Marshall (1992). Immediately after locomotor testing, animals were scored for erectile response, as described in Supplementary Methods.
In situ Hybridization
Animals, treated with nicotine or saline for 1 or 4 days, were decapitated 30 min after the last injection. Brains were frozen in isopentane (−20°C) and stored (−70°C). Cryocut coronal sections (20 μm) were mounted onto coated glass slides and processed for hybridization of c-fos mRNA as described in Winzer-Serhan et al (1999) and detailed in Supplementary Methods.
Ligand Binding Autoradiography
Nicotine- and saline-pretreated early adolescent (P32) and adult (P90) animals were decapitated 24 h after the last pretreatment. Brains were frozen in isopentane and stored (−70°C) until cryostat sectioning. Tissue was processed for transporter binding using methods similar to Pradhan et al (2002). Sections for DAT binding were incubated for 2 h with 10 pM [125I]RTI-55 (Perkin-Elmer NEN, Waltham, MA) in buffer containing 10 mM Na phosphate, 0.1 M sucrose, with 50 nM citalopram HBr, and 5 nM desipramine to block binding to SERT and NET, respectively. Nonspecific binding was determined in adjacent tissue sections by the addition of GBR 12 909 (10 μM). SERT binding conditions were identical to DAT, except that 50 nM citalopram was replaced with 1 μM GBR 12 935 to block binding to DAT, and nonspecific binding was defined by 50 nM citalopram. Sections were rinsed in ice-cold phosphate buffer. After a brief dip in ice-cold water, slides were dried and apposed to Kodak Biomax MR film with autoradiographic standards for 48 (SERT) or 72 h (DAT) in light-proof X-ray cassettes. Film was developed and rapidly fixed. Sections were postfixed with 4% paraformaldehyde and then processed for Nissl staining using cresyl violet for anatomical analysis.
Autoradiography Analysis
Images were analyzed with a computer-based image analysis system (MCID, Imaging Research, St Catherine, Ontario, Canada) using calibrated [14C] brain paste standards of reference. A calibration curve of optical density against radioligand concentration (d.p.m./mg tissue) was constructed (Winzer-Serhan and Leslie, 1997). Analysis was performed by delineating standard geometric shapes around the appropriate anatomical regions. Brain areas for analysis were identified with well-defined anatomical landmarks and with reference to adjacent brain section processed for Nissl-stained sections (Paxinos and Watson, 2007). Optical densities were measured in 2–3 sections in both hemispheres and values of radioactivity were calculated by interpolation from the calibration curve and averaged to give the value for a single region. Specific hybridization was calculated by subtracting values of radioactivity in sections hybridized with sense probe from those hybridized with antisense. Specific radioligand binding was calculated by subtracting values of radioactivity in sections incubates with excess competing ligand from those that were not.
Tissue Catecholamine Levels
Nicotine- and saline-pretreated early adolescent (P32) and adult (P90) animals were euthanized by decapitation 24 h after the last pretreatment. Brains were rapidly removed and dissected on an ice-chilled brain matrix (Plastics One, Roanoke, VA). From 2 mm sections, bilateral 1 mm diameter punches were taken from PFC, dorsal striatum (DS), NAc, and amygdala and expelled into 300 ml of ice-cold 0.1 M perchloric acid, and then homogenized. Samples were centrifuged at 10 000 g for 10 min. The supernatants were used for the measurement of DA, 5-HT, and metabolites using HPLC-ED as described in Cao et al (2007b) and Supplementary Methods, and pellets were used to measure protein content (Bio-Rad, Hercules, CA).
Statistics
All data are expressed as mean±SEM. Self-administration data were analyzed for total reinforced and non-reinforced day 1 responses by two-way ANOVA for age × pretreatment with repeated measures. Reinforced and non-reinforced comparisons were made by paired t-tests. The 30 min session of locomotor activity were divided into six blocks of 5 min and analyzed using a four-way ANOVA for age × pretreatment × drug × time, with repeated measures on time. Antagonist studies were analyzed by three-way ANOVA for antagonist × pretreatment × drug. In situ hybridization data were analyzed by three-way ANOVA for age × pretreatment × duration. Radioligand binding and tissue punch data were analyzed by three-way ANOVA for age × pretreatment × brain area. Following a finding of overall significance, individual brain regions were examined using a two-way ANOVA on age × pretreatment. All significant main or interaction effects were further analyzed by one-way ANOVA with Bonferroni-adjusted post hoc comparisons.
RESULTS
Nicotine Pretreatment Enhances Adolescent Acquisition of Cocaine Self-administration
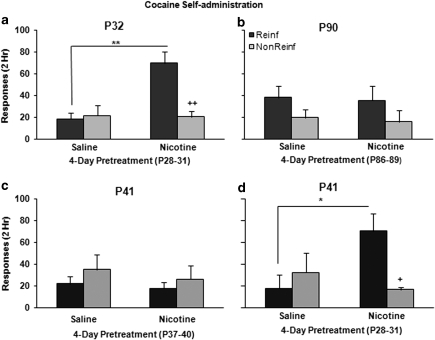
Nicotine pretreatment produced age-dependent changes in reinforced responding for cocaine, as shown by the interaction of reinforcement × age × pretreatment (F(1, 34)=4.47, p=0.04) (Figure 1a and b). Nicotine pretreatment at P28–31 significantly increased reinforced responding for cocaine (F(1, 17)=18.13, p=0.001) at P32 (Figure 1a), and resulted in immediate acquisition of drug self-administration as defined by a significant preference for the cocaine-reinforced over the non-reinforced hole (p=0.007). Nicotine pretreatment in adult rats, aged P86–89, or in older adolescents, aged P37–40, did not significantly affect cocaine intake the following day (Figure 1b and c). In animals pretreated with nicotine on P28–31, cocaine-reinforced responding remained significantly enhanced on P41 (F(1, 8)=6.80, p=0.03), with a significant preference for the reinforced over the non-reinforced hole (p=0.02; Figure 1d). These data indicate that early adolescence is a critical period for nicotine modulation of reward circuitry, and that the effects are evident for at least 10 days. Similar enhancement of initial acquisition of methamphetamine and ethanol self-administration was observed following early adolescent nicotine pretreatment (Supplementary Figure S1).
Figure 1.
Effect of nicotine pretreatment on cocaine self-administration is specific to early adolescence and persists 10 days. Adolescent postnatal day ((P)32, a) and adult (P90, b) animals were given intravenously nicotine or saline daily for 4 days before cocaine (0.5 mg/kg/inf) self-administration tests. The figure shows the mean (±standard error of the mean)-reinforced (dark) and -non-reinforced (light) responses in a 2 h session on day 1. Nicotine-treated P32 rats self-injected significantly more drug than saline-pretreated adolescents or adult animals. They also had greater number of responses at the reinforced compared with non-reinforced hole. Nicotine enhancement of drug intake was not seen when pretreated later in adolescence (P41, c). However, if pretreated in early adolescence, the enhanced self-administration response was still evident when tested 10 days later (P41, d). **p=0.001, *p=0.04 vs P32 saline; ++p<0.01, +p<0.03 reinf vs non-reinf. n=9–10 per P32 and 90 groups. n=5–6 per P41 groups.
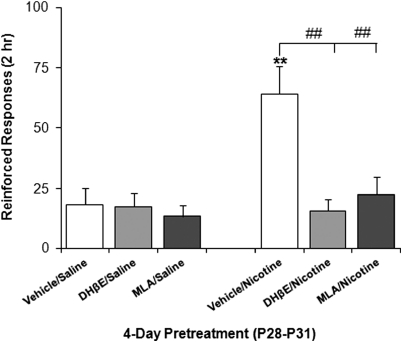
Selective antagonists for α7 and α4β2*nAChR subtypes, MLA (1 mg/kg) and DHβE (2 mg/kg), respectively, administered before daily nicotine pretreatment, blocked nicotine enhancement of cocaine responding with no effect on the behavior of saline-pretreated controls (Figure 2). These data suggest that activation of both major nAChR subtypes is involved in nicotine-induced alterations in adolescents.
Figure 2.
Nicotinic acetylcholine receptor (nAChR) antagonist blockade of pretreatment effect on cocaine self-administration. Adolescent rats were pretreated intravenously with the selective nAChR antagonists, DHβE (3β-1,6-didehydro-14,17-dihydro-3-methoxy-16(15H)-oxaerythrinan-15-one hydrobromide) (2 mg/kg) or MLA (1α,4(S),6β,14α,16β-20-Ethyl-1,6,14,16-tetramethoxy-4-[[[2-(3-methyl-2,5-dioxo-1-pyrrolidinyl)benzoyl]oxy]methyl]aconitane-7,8-diol citrate salt) (1 mg/kg), or vehicle before daily nicotine pretreatment. Nicotine pretreatment enhanced responding for cocaine compared with saline-treated rats, **p<0.005. Pretreatment with either DHβE or MLA blocked the nicotine-enhanced self-administration behavior, ##p<0.003, but had no effect on saline treatment. n=8–10 per group.
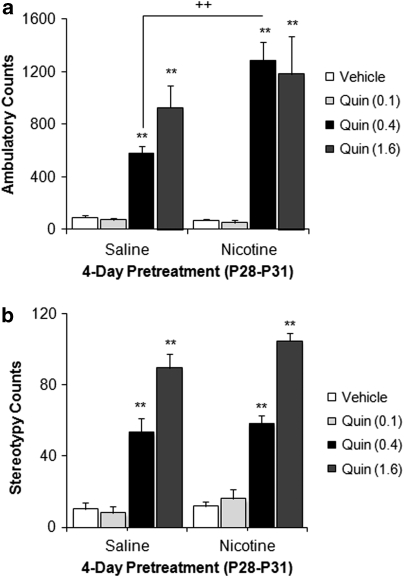
Nicotine Enhances Quinpirole-Induced Locomotion in Adolescent Rats
Figure 3 shows the behavioral response to the D2-like DA agonist, quinpirole, in rats aged P32 following a 4-day nicotine pretreatment. Overall, quinpirole significantly increased locomotor activity (F(3, 78)=69.67, p<0.0001), with a significant pretreatment × quinpirole interaction (F(3, 70)=6.76, p<0.0001). Nicotine-pretreated adolescents showed increased sensitivity to the locomotor-activating effects of a mid-range dose of quinpirole (0.4 mg/kg, intraperitoneal), but not at lower and higher doses (Figure 3a). Although there was a significant effect of quinpirole on stereotypic behavior (F(3, 98)=128.77, p<0.0001)), there was no significant effect of nicotine pretreatment (Figure 3b).
Figure 3.
Effect of age and nicotine pretreatment on quinpirole-induced behavioral responses. Quinpirole (0.4 and 1.6 mg/kg, intraperitoneal) significantly increased locomotor activity (a) and stereotypy (b) in adolescent rats (**p<0.0001). Quinpirole (0.1 mg/kg, intraperitoneal) had no effect on locomotion or stereotypy compared with vehicle controls. Nicotine-pretreated adolescents showed increased locomotion following quinpirole (0.4 mg/kg) injection (a) compared with saline-pretreated controls (++p<0.0001 vs saline pretreated). n=6–13 per group
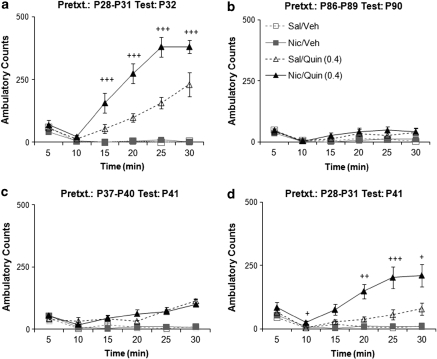
Nicotine pretreatment effects on quinpirole-induced locomotion were age-specific (Figure 4a–d). As has been reported previously (Frantz et al, 1999), quinpirole-induced locomotion in saline-pretreated controls decreased with age (F(1, 67)=6.29, p=0.015). Animals pretreated with nicotine during early adolescence (P28–31) exhibited enhanced quinpirole-induced locomotion at P32 (F(1, 45)=44.03, p<0.0001; Figure 4a) and at P41 (F(1, 22)=11.15, p=0.003; Figure 4b), whereas pretreatment during later adolescence (P37–40) and adulthood (P86–89) had no effect (Figure 4c and d). Thus, nicotine enhancement of quinpirole-induced locomotion is specific to exposure during the early adolescent period (P28–31) and these effects persist at least 10 days.
Figure 4.
Effect of nicotine pretreatment on quinpirole-induced locomotion is specific to early adolescence and persists 10 days. Adolescent postnatal day ((P)32) and adult (P90) animals were given intravenously nicotine or saline daily for 4 days before drug injection. Time course of ambulatory counts during 30 min after drug injection. Nicotine-treated P32 rats showed a significant enhancement of locomotor activity after quinpirole administration compared with saline-treated controls (P28–P31 Test: P32, a). Nicotine enhancement of quinpirole-induced locomotion was not seen when animals were pretreated during adulthood (P86–89 Test: P90, b) or late adolescence (P37–40 Test: P41, c). However, if pretreated in early adolescence, the enhanced quinpirole-induced locomotion was still evident when tested 10 days later (P28–31 Test: P41, d). +++p<0.0001, ++p<0.002, +p=0.01 vs Sal/Quin (0.4). n=6–14 per P32 and 90 groups. n=7–8 per P41 groups.
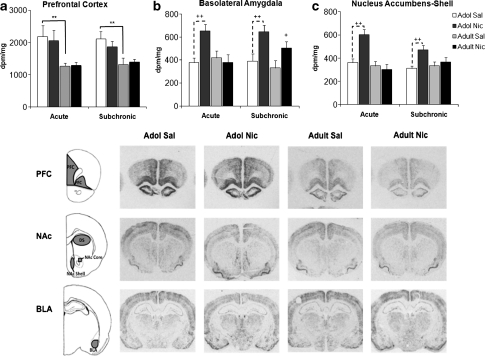
Acute and Subchronic Nicotine Treatment Induces Age-dependent Neural Activation
To identify mechanisms underlying adolescent nicotine pretreatment effects, we examined regional mRNA expression of the immediate-early gene, c-fos, after either acute or subchronic (4-day) nicotine treatment in adolescent and adult rats (Figure 5). Acute nicotine caused a significant, age-dependent increase in c-fos mRNA levels in the BLA and NAc shell (age × nicotine interaction, BLA, F(1, 21)=9.79, p=0.005; NAc shell, F(1, 21)=8.61, p=0.008). In these limbic regions, adolescent animals showed a nicotine-induced neural response that was not observed in adults. Although age differences were seen in basal c-fos expression in PFC (F(1, 21)=8.14, p<0.001), DS (data not shown; F(1, 21)=4.41, p=0.048), and NAc core (data not shown; F(1, 21)=53.09, p<0.001), there was no significant effect of acute nicotine treatment in these regions.
Figure 5.
Age differences in c-fos mRNA activation by acute or subchronic nicotine treatment. Age differences were observed in the prefrontal cortex (a), **p⩽0.02, but there was no effect of nicotine treatment. Solid line brackets denote age effects and dashed brackets represent pretreatment effects. Acute and subchronic nicotine-induced c-fos activation in the basolateral amygdala (BLA) and nucleus accumbens shell (NAc) shell of adolescent rats (b and c), ++p<0.01, whereas adults only show nicotine-induced activation of BLA after subchronic treatment (c), +p<0.05. Schematic representation of brain regions and autoradiographic analysis of regional c-fos mRNA levels (d). The gray regions were analyzed for c-fos. n=4–11 per group.
Nicotine-induced c-fos expression was still evident in adolescent BLA (F(1, 29)=29.44, p<0.001) and NAc shell (F(1, 25)=10.59, p=0.003) on the fourth treatment day. However, age differences were only seen in the NAc shell (age × nicotine interaction; F(1, 25)=4.65, p=0.04), with nicotine-induced neuronal activation observed only in adolescent animals. Following subchronic nicotine treatment, increased c-fos mRNA expression was seen in the BLA of both adolescents and adults. Although significant age differences in basal c-fos expression were seen in the PFC and NAc core repeated treatment groups (PFC, F(1, 24)=11.02, p=0.003; NAc core (data not shown) (1, 30)=4.20, p=0.049), there was no significant effect of subchronic nicotine.
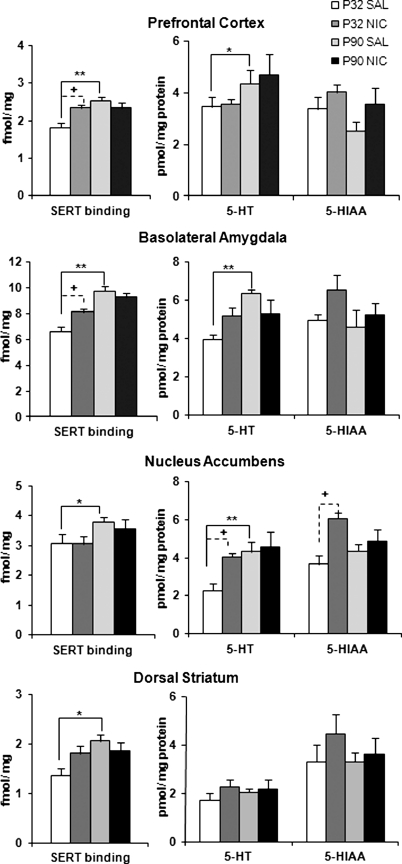
Adolescent Nicotine Pretreatment Alters Monoamines in Mesolimbic Reward Circuitry
The effects of subchronic nicotine treatment on presynaptic DA and 5-HT markers were assessed in the PFC, NAc, DS, and BLA (Table 1 and Figure 6; Supplementary Figures S3 and S4). Serotonergic markers showed both age- and treatment-specific changes. Age differences in SERT binding were observed in the PFC (F(1, 31)=9.66, p=0.004), NAc (F(1, 31)=5.55, p=0.025), DS (F(1, 31)=6.36, p=0.017), and BLA (F(1, 31)=32.00, p<0.001), with post hoc comparisons showing lower SERT density in adolescent controls than adults (PFC, p=0.001; DS, p=0.017; BLA, p<0.001; NAc, NS). Significant age–pretreatment interactions were found for the PFC (F(1, 31)=9.92, p=0.004), BLA (F(1, 31)=8.92, p=0.005), and DS (F(1, 31)=4.59, p=0.04), with post hoc comparisons showing significant nicotine-induced increases in SERT binding in adolescents but not adults (PFC, p=0.02; BLA, p=0.013, DS, NS).
Table 1. Effect of 4-Day Nicotine Pretreatment on Regional DAT Binding and DA and DOPAC Content.
| Region | Marker | P32 saline | P32 nicotine | P90 saline | P90 nicotine |
|---|---|---|---|---|---|
| PFC | DA | 0.49±0.06* | 0.55±0.04* | 0.77±0.13 | 0.80±0.14 |
| DOPAC | 0.09±0.04 | 0.13±0.03 | 0.10±0.04 | 0.2±0.06 | |
| NAc | DAT | 0.57±0.04 | 0.59±0.04 | 0.56±0.01 | 0.54±0.04 |
| DA | 41.84±6.99 | 60.04±4.26† | 42.72±6.27 | 52.53±1.68† | |
| DOPAC | 6.43±1.05 | 9.93±1.28 | 6.61±1.58 | 6.99±0.75 | |
| BLA | DAT | 0.16±0.01* | 0.18±0.01 | 0.22±0.02 | 0.19±0.02 |
| DA | 26.71±4.91 | 20.73±3.67 | 28.76±3.97 | 21.02±5.69 | |
| DOPAC | 2.05±0.46 | 2.11±0.48 | 2.07±0.35 | 1.63±0.35 |
Adolescent and adult animals received a 4-day pretreatment of nicotine or saline. At 24 h after the last infusions, brains were collected and sectioned for radioligand binding with 125IRTI-55 or tissue punches were collected for the analysis of catecholamine content. Adolescent animals had lower DAT density of the BLA as compared with adults, *p<0.05. Overall age differences were also seen with DA content in the PFC/(*p=0.01). Overall nicotine pretreatment significantly increased DA in the NAc (†p=0.01). Transporter binding, n=7–10 per group; tissue punches, n=6–7 per group.
Figure 6.
Effect of 4-day nicotine pretreatment on regional serotonin transporter (SERT) binding and serotonin (5-HT) and 5-hydroxyindoleacetic acid (5-HIAA) content. Adolescent and adult animals received a 4-day pretreatment of nicotine (0.06 mg/kg, intravenous) or saline, and brains were removed 24 h after the last infusion, and sectioned for radioligand binding with [125I]RTI-55 or tissue punches were collected for analysis of monoamine content. Solid line brackets denote age effects and dashed brackets represent pretreatment effects. Age differences in SERT were observed in all regions, *p<0.03, **p<0.01 vs postnatal day (P)90Sal. Adolescent nicotine pretreatment increased SERT density in the prefrontal cortex (PFC) and basolateral amygdala (BLA), +p<0.01 vs P32Sal. For 5-HT content, PFC, BLA, and nucleus accumbens (NAc) showed a significant overall age effect (*p=0.05 overall, **p<0.01 vs P90Sal). Adolescent nicotine pretreatment enhanced 5-HT and 5-HIAA levels in the NAc; +p⩽0.05. Transporter binding, n=7–10 per group; tissue punches, n=6–7 per group.
Significant age differences were found for 5-HT content in PFC (F(1, 21)=4.22, p=0.05), NAc (F(1, 21)=9.89, p=0.005), and BLA (F(1, 21)=7.70, p=0.01), with post hoc comparisons showing significantly lower levels in adolescent controls compared with adults (PFC, NS; NAc, p=0.014; BLA, p=0.008). Significant nicotine pretreatment effects were observed in NAc for both 5-HT (F(1, 21)=6.40, p=0.02) and 5-HIAA (F(1, 21)=6.20, p=0.02), with adolescent nicotine treatment increasing tissue levels as compared with saline controls (5-HT, p=0.03; 5-HIAA, p=0.04). There was a significant age × pretreatment interaction (F(1, 21)=6.62, p=0.02) for 5-HT levels in the BLA.
Age differences were seen for DA markers, with adolescents exhibiting significantly lower DAT density in BLA and lower DA content in PFC and DS compared with adult controls. Although nicotine pretreatment did increase NAc DA levels, this effect was not age specific. A more detailed description of DA results can be found in the Supplementary Materials.
Involvement of 5-HT1A Receptors in Nicotine-Enhanced Behaviors
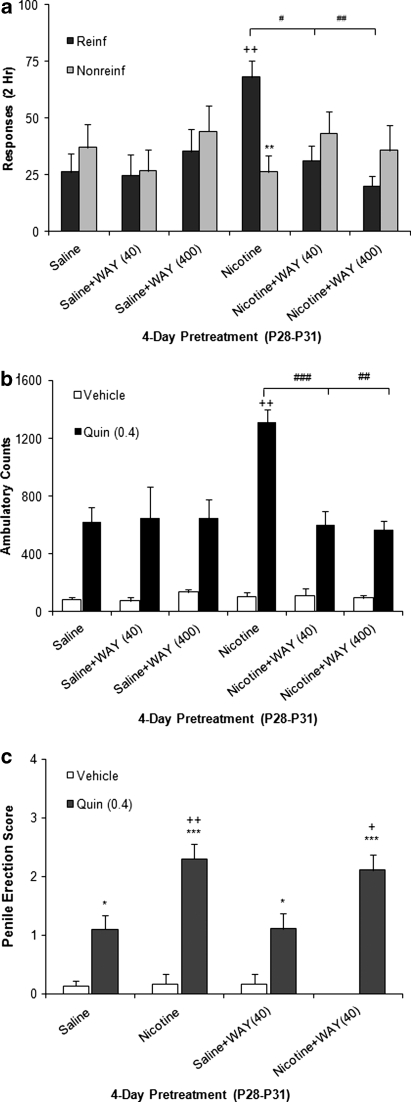
To determine whether 5-HT1A-Rs mediate nicotine pretreatment effects in adolescents, WAY 100 635 (40 and 400 μg/kg), was co-administered during nicotine pretreatment. Animals were subsequently tested for acquisition of cocaine self-administration (Figure 7a) or locomotor activity and erectile response after quinpirole challenge (0.4 mg/kg intraperitoneal; Figure 7b and c).
Figure 7.
Effect of serotonin 1A receptor (5-HT1A-R) antagonism on behavioral enhancement seen after adolescent nicotine pretreatment. Adolescent animals received daily infusions of WAY 100 635 (N-{2-[4-(2-methoxyphenyl)-1-piperazinyl] ethyl}-N-(2-pyridinyl)cyclohexanecarboxamide) (0, 40, or 400 μg/kg, intravenous) in combination with nicotine pretreatment. WAY blocked the nicotine pretreatment effect on cocaine 1 (0.5 mg/kg/inf; a) intake and acquisition on day 1 and quinpirole-induced locomotion (0.4 mg/kg, intraperitoneal; b). ++p=0.001 vs saline. ###p<0.0001, ##p⩽0.001 vs nicotine. Administration of WAY had no effect on nicotine enhancement of penile erection in response to quinpirole (c). ***p<0.0001, *p<0.5 vehicle; ++p<0.001vs saline/quin (0.4); +p<0.05 vs saline+WAY (400). Cocaine: n=8–12 per group and quinpirole: n=4–8 per group.
5-HT1A-R antagonist pretreatment significantly decreased cocaine-reinforced responding (F(2, 63)=4.22, p=0.02) and yielded a significant interaction with pretreatment (F(2, 63)=6.90, p=0.002). Although nicotine pretreatment enhanced cocaine responding (p=0.002), WAY 100 635 pretreatment blocked this nicotine effect, but did not alter behavior of saline-pretreated controls (Figure 7a). A similar effect of 5-HT1A-R blockade was found for nicotine enhancement of adolescent methamphetamine self-administration (Supplementary Figure S4). These findings indicate that nicotine pretreatment enhances initial psychostimulant reward by 5-HT1A-R stimulation.
WAY 100 635 also significantly blocked nicotine enhancement of adolescent quinpirole-induced locomotion (F(11, 72)=11.01, P<0.0001), with a significant interaction of pretreatment (F(5, 72)=3.12, p=0.014). Although nicotine pretreatment enhanced quinpirole-induced locomotion (p=0.002), co-administration of WAY 100 635 blocked this nicotine effect (40 μg/kg dose, p<0.0001; 400 μg/kg dose, p=0.001). 5-HT1A-R antagonist pretreatment did not alter the behavior of saline-pretreated controls.
Quinpirole significantly increased penile erection exclusively in adolescents (Supplementary Figure S5). In examining erectile response, there was a significant interaction of pretreatment and drug (F(5, 72)=3.39, p=0.024) (Figure 7c). Adolescent nicotine exposure significantly altered quinpirole-induced erectile response (F(3, 62)=3.38; p=0.025). Adolescents pretreated with nicotine showed a significant enhancement of quinpirole-induced penile erection (p=0.004), which was not blocked by WAY 100 635 (40 μg/kg). These findings suggest that two different mechanisms underlie nicotine enhancement of adolescent DA-mediated behaviors: a 5-HT1A-R-mediated pathway for cocaine self-administration and quinpirole locomotion, and a distinct pathway mediating the potentiation of erectile response.
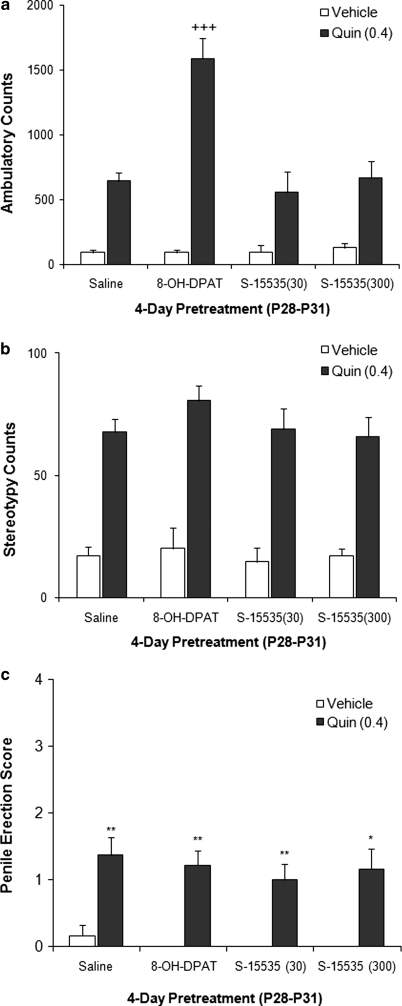
Pretreatment with specific 5-HT1A agonists further elucidated the contribution of 5-HT1A-Rs to nicotine-induced enhancement of quinpirole-induced locomotor activity (Figure 8). There was an overall interaction of pretreatment × quinpirole × age ((F(1, 59)=9.407, p=0.003)). Pretreatment with 8-OH-DPAT (10 μg/kg, intravenous), an agonist that activates both pre- and post-synaptic 5-HT1A-Rs (Larsson et al, 1990), enhanced quinpirole-induced locomotion in adolescents (F(1, 20)=14.57, p=0.001). As it is unclear if the effective doses of WAY 100 635 and 8-OH-DPAT used in this study preferentially target autoreceptors or if they target both autoreceptor and heteroceptors, studies were undertaken using the selective 5-HT1A-R ligand, S-15535, which is an agonist at autoreceptors and an antagonist at heteroceptors (Millan et al, 1994; Newman-Tancredi et al, 1999). Pretreatment with the selective 5-HT1A autoreceptor agonist, S-15535 (30 or 300 μg/kg intravenous), had no effect on quinprole-induced locomotion (F(2, 49)=0.633, p=0.535) or stereotypy (F(2, 49)=0.069, p=0.933). Furthermore, 8-OH-DPAT pretreatment did not enhance quinpirole-induced stereotypy or penile erection (Figure 8b and c). These data suggest the involvement of 5-HT1A-R heteroceptors in adolescent nicotine enhancement of quinpirole-induced locomotion, and the involvement of a non-5-HT1A receptor-mediated mechanism for the enhancement of erectile response.
Figure 8.
Effect of direct serotonin 1A receptor (5-HT1A-R) agonist pretreatment on quinpirole-induced behaviors. Adolescent animals received daily injections of 8-OH-DPAT ((+/−)-8-hydroxy-2-(di-n-propylamino)tetralin) (10 μg/kg) or S-15535 (1-(2,3-dihydro-1,4-benzodioxin-5-yl)-4-(2,3-dihydro-1h-inden-2-yl)-piperazine) (30 or 300 μg/kg) for 4 days postnatal day (P)28–31). (a) 8-OH-DPAT-pretreated adolescents showed enhanced quinpirole (0.4 mg/kg)-induced locomotion compared with saline pretreatment controls (+++p<0.0001 vs saline). Pretreatment with S-15535 (30 and 300 μg/kg), a presynaptic agonist did not enhance quinpirole-induced locomotion. (b) Although there was a drug effect for stereotypy for 8-OH-DPAT- and S-15535-pretreated animals, there were no effects of pretreatment. n=6–12 per group. (c) Quinpirole induced an erectile response in adolescent animals. *p<0.5, **p<0.001 vs vehicle. However, 8-OH-DPAT and S-15535 pretreatment did not enhance quinpirole-induced penile erection. n=4–9.
DISCUSSION
We have shown that brief treatment with a low dose of nicotine induces unique functional changes in adolescent drug response, with increased initial self-administration of cocaine, methamphetamine, and alcohol, and enhanced locomotor and erectile response to the D2-like agonist, quinpirole. These functional changes are long lasting, being evident 10 days after the last nicotine treatment, and are highly age dependent. Increased responding was observed when nicotine pretreatment occurred during early adolescence (P28–31), but not during late adolescence (P38–41) or adulthood (P86–89).
Using c-fos mRNA expression to identify recently activated neural circuits, we found unique activating effects of nicotine on adolescent NAc shell and BLA. Furthermore, although neurochemical analyses showed that both dopaminergic and serotonergic inputs to mesocorticolimbic regions change dynamically during this developmental period, adolescent nicotine pretreatment had more striking effects on normative 5-HT function, with increases in SERT density in PFC and BLA, and in 5-HT levels in BLA and NAc. Pharmacological studies confirmed an involvement of 5-HT systems in mediating the effects of adolescent nicotine pretreatment on drug reinforcement and locomotor activity. Nicotine enhancement of cocaine and methamphetamine self-administration, and of quinpirole-induced locomotor activity, was blocked by co-administration of WAY 100 635, a selective 5-HT1A receptor antagonist. Furthermore, adolescent pretreatment with the 5-HT1A receptor agonist, 8-OH-DPAT, but not the autoreceptor selective agonist, S-15535, enhanced quinpirole-induced locomotor activation, but not stereotypy or penile erection. These findings indicate that early adolescent nicotine exposure uniquely alters limbic function by both 5-HT1A and non-5-HT1A mechanisms. Although 5-HT1A heteroceptor activation appears to underlie nicotine enhancement of drug reinforcement and locomotion, another, as yet unknown, mechanism underlies the enhancement of quinpirole-induced erectile response.
Adolescent Maturation of the Mesocorticolimbic System
An extensive literature suggests that acetylcholine activation of nAChRs regulates the maturation of neuronal circuitry during sensitive developmental periods (Dwyer et al, 2009). This finding of early adolescence as a critical period of vulnerability to the effects of nicotine is consistent with other recent animal studies that have shown unique effects of nicotine at this age (Azam et al, 2007; Adriani et al, 2003; Belluzzi et al, 2004; Park et al, 2007; Kota et al, 2007). Furthermore, the actively maturing PFC–NAc–BLA triadic circuit appears to be highly sensitive to the effects of early adolescent nicotine exposure. Our data confirm and extend a previous finding that adolescents have greater nicotine-induced activation in the NAc shell (Shram et al, 2007). Although acute nicotine does not induce c-fos in BLA of adult animals (Cao et al, 2007a; Valjent et al, 2002; Mathieu-Kia et al, 1998), we now show an adolescent-specific nicotine-induced activation of the BLA.
In agreement with previous findings of late maturation of DA systems (Cao et al, 2007b; Chambers et al, 2003; Chen et al, 1997), we found lower DAT levels in the BLA and lower DA levels in the PFC and DS of adolescents as compared with adults. Although nicotine pretreatment did increase DA levels in the NAc, this effect was not age dependent and therefore did not underlie the observed adolescent-specific functional changes. As has been shown previously (Frantz et al, 1999), we found that locomotor activity induced by the D2-like agonist, quinpirole, declined with age. We now also show a similar age-related decline in quinpirole-induced erectile response. Such findings are consistent with major developmental alterations of D2-like receptor function during adolescence (Chen et al, 2010). Nicotine pretreatment during the early adolescent period produced an extended enhancement of these functional responses to quinpirole, suggesting an alteration of D2-like receptor signaling.
Whereas others have reported an early maturation of 5-HT content and SERT density (Chambers et al, 2003; Chen et al, 1997; Moll et al, 2000), we found adolescents to have lower 5-HT content in PFC, NAc, and BLA, and lower SERT levels in all regions examined. Such findings are consistent, however, with transient declines in 5-HT synapses that have been noted in other brain regions during postnatal development (Dinopoulos et al, 1997; Dori et al, 1998). Our present finding that nicotine induces age-specific changes in 5-HT markers within the adolescent limbic system is consistent with the findings of a recent in vivo microdialysis study in which acute nicotine was reported to enhance monoamine release in this region in adolescents but not adults (Shearman et al, 2008). These data support the concept that normative function of 5-HT within the PFC–NAc–BLA circuit is altered by early adolescent nicotine exposure.
Receptor Mechanisms Underlying Adolescent Nicotine Effects
Using a pharmacological approach, we have identified a critical role for 5-HT1A receptors in nicotine regulation of adolescent limbic pathways. Chronic nicotine exposure during adolescence has been previously reported to increase 5-HT1A-R binding (Slotkin et al, 2007). These receptors are located in limbic regions, such as NAc and BLA (Pazos and Palacios, 1985), in which adolescent nicotine pretreatment induced changes in 5-HT markers. As the 5-HT1A-R antagonist WAY 100 635 has significant affinities for other receptors (Fletcher et al, 1995), we also determined whether selective agonists could mimic the effects of adolescent nicotine pretreatment. Enhancement of quinpirole-induced locomotion resulted from pretreatment with 8-OH-DPAT, a mixed autoreceptor/heteroceptor agonist (Larsson et al, 1990), but not S-15535, an autoreceptor-specific agonist (Millan et al, 1993). Such findings suggest that early adolescent nicotine enhancement of initial drug self-administration and quinpirole-induced locomotion is mediated by 5-HT release and activation of 5-HT1A-R heteroceptors. In contrast, WAY 100 635 and 8-OH-DPAT pretreatment did not influence erectile response, confirming a different mechanism for this nicotine effect.
Although nicotine may induce biological effects by either activation or desensitization of nAChRs (Tapper et al, 2004; Pidoplichko et al, 1997), our nAChR antagonist data suggest that activation of each of the two major nAChR classes, α7 and α4β2*, underlies nicotine enhancement of drug-reinforced behavior in adolescents. Both α7 and α4β2* nAChRs are located on DA and 5-HT cell bodies in the ventral tegmental area and dorsal raphe, respectively, and their stimulation can enhance the release of monoamines in terminal fields such as PFC, NAc, and BLA (Azam et al, 2007; Mihailescu et al, 1998; Galindo-Charles et al, 2008; Wonnacott et al, 2005). It is not clear from this study whether the effect of nicotine on adolescent limbic function that we have observed results from a transient appearance of these nAChRs or from an increase in drug sensitivity. Further studies with higher doses of nicotine will be needed to address this issue.
Clinical Implications
There are high rates of co-morbidity of smoking and other substance abuse disorders, including psychostimulant addiction (Weinberger and Sofuoglu, 2009) and alcoholism (Grucza and Bierut, 2006). Although such co-dependence may reflect genetic or social factors, several clinical studies have suggested that adolescent smoking may increase the subsequent use of other addictive substances (Grucza and Bierut, 2006; Torabi et al, 1993; Lai et al, 2000; Biederman, 2005; Palmer et al, 2009). Our findings provide evidence that nicotine administration during adolescence causes long-lasting behavioral and neurochemical adaptations, and elucidate novel pathways altered by nicotine that may increase vulnerability to high-risk behaviors. Although future studies must be performed to identify the specific roles of each neural area, we have shown that early adolescence is a sensitive period for the proper formation of the PFC–BLA–NAc circuit, and that environmental influences may produce extended changes in its function, with important consequences for mental health-related behaviors. In particular, adolescents are exposed to drugs—both clinical and recreational—that may interfere with limbic circuitry development, according to our model. Activation of nAChRs by early adolescent smoking may induce changes in DA receptor signaling and initial drug reinforcement, primarily by activation of 5-HT1A-Rs. However, pathways underlying sexual behavior may be enhanced by nicotine by other neurochemical mechanisms. Our findings also suggest that early adolescent treatment with SERT inhibitor antidepressant therapies may result in the activation of 5-HT1A-Rs (El Mansari and Blier, 2005; Savitz et al, 2009), with subsequent alterations in limbic function. Such changes may be associated with the clinical observation that these antidepressant therapies are less effective in adolescents than in adults (Hammad et al, 2006; Olfson et al, 2006; Bridge et al, 2007).
Acknowledgments
This work was supported by PHS Grant DA 19138. We thank YiLing Chen, Celina Mojica, and Jenny H Lee for their excellent technical support.
The authors declare no conflicts of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Abreu-Villaca Y, Seidler FJ, Qiao D, Tate CA, Cousins MM, Thillai I, et al. Short-term adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: critical Periods, patterns of exposure, dose thresholds. Neuropsychopharmacology. 2003;28:1935–1949. doi: 10.1038/sj.npp.1300221. [DOI] [PubMed] [Google Scholar]
- Abreu-Villaça Y, Filgueiras CC, Guthierrez M, Medeiros AHD, Mattos MA, Pereira MDS, et al. Exposure to tobacco smoke containing either high or low levels of nicotine during adolescence: differential effects on choline uptake in the cerebral cortex and hippocampus. Nicotine Tob Res. 2010;12:776–780. doi: 10.1093/ntr/ntq075. [DOI] [PubMed] [Google Scholar]
- Adriani W, Granstrem O, Macri S, Izykenova G, Dambinova S, Laviola G. Behavioral and neurochemical vulnerability during adolescence in mice: studies with nicotine. Neuropsychopharmacology. 2004;29:869–878. doi: 10.1038/sj.npp.1300366. [DOI] [PubMed] [Google Scholar]
- Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, et al. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci. 2003;23:4712–4716. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rustein M, Hostetter JC, Teigher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Azam L, Chen Y, Leslie FM. Developmental regulation of nicotinic acetylcholine receptors within midbrain dopamine neurons. Neuroscience. 2007;144:1347–1360. doi: 10.1016/j.neuroscience.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdy G, To CT. Comparison of relative potencies of i.v. and i.c.v. administered 8-OH-DPAT gives evidence of different sites of action for hypothermia, lower lip retraction and tail flicks. Eur J Pharmacol. 1997;323:53–58. doi: 10.1016/s0014-2999(97)00021-6. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology. 2004;174:389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Benoit-Marand M, O'Donnell P. D2 dopamine modulation of corticoaccumbens synaptic responses changes during adolescence. Eur J Neurosci. 2008;27:1364–1372. doi: 10.1111/j.1460-9568.2008.06107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry. 2005;57:1215–1220. doi: 10.1016/j.biopsych.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Mick E, Wilens TE, Fontanella JA, Poetzl KM, et al. Is cigarette smoking a gateway to alcohol and illicit drug use disorders? A study of youths with and without attention deficit hyperactivity disorder. Biol Psychiatry. 2006;59:258–264. doi: 10.1016/j.biopsych.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Biglan A, Metzler CW, Wirt R, Ary D, Noell J, Ochs L, et al. Social and behavioral factors associated with high-risk sexual behavior among adolescents. J Behav Med. 1990;13:245–261. doi: 10.1007/BF00846833. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci Off J Soc Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge JA, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus HA, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297:1683–1696. doi: 10.1001/jama.297.15.1683. [DOI] [PubMed] [Google Scholar]
- Bronisch T, Höfler M, Lieb R. Smoking predicts suicidality: findings from a prospective community study. J Affect Disord. 2008;108:135–145. doi: 10.1016/j.jad.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Brook JS, Balka EB, Ning Y, Brook DW. Trajectories of cigarette smoking among African Americans and Puerto Ricans from adolescence to young adulthood: associations with dependence on alcohol and illegal drugs. Am J Addict/Am Acad Psychiatrists Alcohol Addict. 2007;16:195–201. doi: 10.1080/10550490701375244. [DOI] [PubMed] [Google Scholar]
- Cao J, Belluzzi JD, Loughlin SE, Keyler DE, Pentel PR, Leslie FM. Acetaldehyde, a major constituent of tobacco smoke, enhances behavioral, endocrine, and neuronal responses to nicotine in adolescent and adult rats. Neuropsychopharmacology. 2007a;32:2025–2035. doi: 10.1038/sj.npp.1301327. [DOI] [PubMed] [Google Scholar]
- Cao J, Lotfipour S, Loughlin SE, Leslie FM. Adolescent maturation of cocaine-sensitive neural mechanisms. Neuropsychopharmacology. 2007b;32:2279–2289. doi: 10.1038/sj.npp.1301349. [DOI] [PubMed] [Google Scholar]
- Carey RJ, DePalma G, Damianopoulos E. Cocaine and serotonin: a role for the 5-HT(1A) receptor site in the mediation of cocaine stimulant effects. Behav Brain Res. 2001;126:127–133. doi: 10.1016/s0166-4328(01)00253-4. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YI, Choi J, Xu H, Ren J, Andersen SL, Jenkins BG. Pharmacologic neuroimaging of the ontogeny of dopamine receptor function. Dev Neurosci. 2010;32:125–138. doi: 10.1159/000286215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Turiak G, Galler J, Volicer L. Postnatal changes of brain monoamine levels in prenatally malnourished and control rats. Int J Dev Neurosci. 1997;15:257–263. doi: 10.1016/s0736-5748(96)00121-9. [DOI] [PubMed] [Google Scholar]
- Covey LS, Manubay J, Jiang H, Nortick M, Palumbo D. Smoking cessation and inattention or hyperactivity/impulsivity: a post hoc analysis. Nicot Tob Res. 2008;10:1717–1725. doi: 10.1080/14622200802443536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinopoulos A, Dori IE, Parnavelas JG. The serotonin innervation of the basal forebrain shows a transient phase during development. Brain Res Dev Brain Res. 1997;99:38–52. doi: 10.1016/s0165-3806(96)00198-8. [DOI] [PubMed] [Google Scholar]
- Dori IE, Dinopoulos A, Parnavelas JG. The development of the synaptic organization of the serotonergic system differs in brain areas with different functions. Exp Neurol. 1998;154:113–125. doi: 10.1006/exnr.1998.6937. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol Therap. 2009;122:125–139. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mansari M, Blier P. Responsiveness of 5-HT(1A) and 5-HT2 receptors in the rat orbitofrontal cortex after long-term serotonin reuptake inhibition. J Psychiatry Neurosci. 2005;30:268–274. [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neurosci Biobehav Rev. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher A, Forster EA, Bill DJ, Brown G, Cliffe IA, Hartley JE, et al. Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav Brain Res. 1995;73:337–353. doi: 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5-HT(2A) receptor antagonist M100907 and the 5-HT(2C) receptor antagonist SB242084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology. 2002;27:576–586. doi: 10.1016/S0893-133X(02)00342-1. [DOI] [PubMed] [Google Scholar]
- Frantz KJ, O'Dell LE, Parsons LH. The locomotor effects of quinpirole in rats depend on age and gender. Pharmacol Biochem Behav. 1999;64:821–826. doi: 10.1016/s0091-3057(99)00162-8. [DOI] [PubMed] [Google Scholar]
- Galindo-Charles L, Hernandez-Lopez S, Galarraga E, Tapia D, Bargas J, Garduño J, et al. Serotoninergic dorsal raphe neurons possess functional postsynaptic nicotinic acetylcholine receptors. Synapse. 2008;62:601–615. doi: 10.1002/syn.20526. [DOI] [PubMed] [Google Scholar]
- Gehricke J, Loughlin SE, Whalen CK, Potkin SG, Fallon JH, Jamner LD, et al. Smoking to self-medicate attentional and emotional dysfunctions. Nicotine Tob Res. 2007;9 (Suppl 4:S523–S536. doi: 10.1080/14622200701685039. [DOI] [PubMed] [Google Scholar]
- Grucza RA, Bierut LJ. Co-occurring risk factors for alcohol dependence and habitual smoking: update on findings from the Collaborative Study on the Genetics of Alcoholism. Alcohol Res Health. 2006;29:172–178. [PMC free article] [PubMed] [Google Scholar]
- Haddjeri N, Lavoie N, Blier P. Electrophysiological evidence for the tonic activation of 5-HT(1A) autoreceptors in the rat dorsal raphe nucleus. Neuropsychopharmacology. 2004;29:1800–1806. doi: 10.1038/sj.npp.1300489. [DOI] [PubMed] [Google Scholar]
- Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63:332–339. doi: 10.1001/archpsyc.63.3.332. [DOI] [PubMed] [Google Scholar]
- Isensee B, Wittchen H, Stein MB, Höfler M, Lieb R. Smoking increases the risk of panic: findings from a prospective community study. Arch Gen Psychiatry. 2003;60:692–700. doi: 10.1001/archpsyc.60.7.692. [DOI] [PubMed] [Google Scholar]
- John U, Meyer C, Rumpf H, Hapke U. Smoking, nicotine dependence and psychiatric comorbidity—a population-based study including smoking cessation after three years. Drug Alcohol Depend. 2004;76:287–295. doi: 10.1016/j.drugalcdep.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Yamaguchi K, Chen K. Stages of progression in drug involvement from adolescence to adulthood: further evidence for the gateway theory. J Stud Alcohol. 1992;53:447–457. doi: 10.15288/jsa.1992.53.447. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Therap. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- LaHoste GJ, Marshall JF. Dopamine supersensitivity and D1/D2 synergism are unrelated to changes in striatal receptor density. Synapse. 1992;12:14–26. doi: 10.1002/syn.890120103. [DOI] [PubMed] [Google Scholar]
- Lai S, Lai H, Page JB, McCoy CB. The association between cigarette smoking and drug abuse in the United States. J Addict Dis. 2000;19:11–24. doi: 10.1300/J069v19n04_02. [DOI] [PubMed] [Google Scholar]
- Lam TH, Stewart SM, Ho LM. Smoking and high-risk sexual behavior among young adults in Hong Kong. J Beh Med. 2001;24:503–518. doi: 10.1023/a:1012227728232. [DOI] [PubMed] [Google Scholar]
- Larsson LG, Rényi L, Ross SB, Svensson B, Angeby-Möller K. Different effects on the responses of functional pre- and postsynaptic 5-HT1A receptors by repeated treatment of rats with the 5-HT1A receptor agonist 8-OH-DPAT. Neuropharmacology. 1990;29:85–91. doi: 10.1016/0028-3908(90)90047-u. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Rohde P, Brown RA. Level of current and past adolescent cigarette smoking as predictors of future substance use disorders in young adulthood. Addiction. 1999;94:913–921. doi: 10.1046/j.1360-0443.1999.94691313.x. [DOI] [PubMed] [Google Scholar]
- Mathieu-Kia AM, Pages C, Besson MJ. Inducibility of c-Fos protein in visuo-motor system and limbic structures after acute and repeated administration of nicotine in the rat. Synapse. 1998;29:343–354. doi: 10.1002/(sici)1098-2396(199808)29:4<343::aid-syn6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- McQuown SC, Belluzzi JD, Leslie FM. Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotoxicol Teratol. 2007;29:66–73. doi: 10.1016/j.ntt.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuown SC, Dao JM, Belluzzi JD, Leslie FM. Age-dependent effects of low-dose nicotine treatment on cocaine-induced behavioral plasticity in rats. Psychopharmacology. 2009;207:143–152. doi: 10.1007/s00213-009-1642-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihailescu S, Palomero-Rivero M, Meade-Huerta P, Maza-Flores A, Drucker-Colín R. Effects of nicotine and mecamylamine on rat dorsal raphe neurons. Eur J Pharmacol. 1998;360:31–36. doi: 10.1016/s0014-2999(98)00658-x. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Rivet JM, Gobert A, Canton H, Veiga S, Bervoets K, et al. 5-HT1A receptors and the tail-flick response. VI. Intrinsic alpha 1A-adrenoceptor antagonist properties can mask the actions of 5-HT1A receptor agonists in the spontaneous tail-flick paradigm. J Pharmacol Exp Therap. 1994;269:121–131. [PubMed] [Google Scholar]
- Millan MJ, Rivet J, Canton H, Lejeune F, Gobert A, Widdowson P, et al. S 15535: a highly selective benzodioxopiperazine 5-HT1A receptor ligand which acts as an agonist and an antagonist at presynaptic and postsynaptic sites respectively. Eur J Pharmacol. 1993;230:99–102. doi: 10.1016/0014-2999(93)90416-f. [DOI] [PubMed] [Google Scholar]
- Moll GH, Mehnert C, Wicker M, Bock N, Rothenberger A, Rüther E, et al. Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile life to late adulthood. Dev Brain Res. 2000;119:251–257. doi: 10.1016/s0165-3806(99)00182-0. [DOI] [PubMed] [Google Scholar]
- Murrin LC, Sanders JD, Bylund DB. Comparison of the maturation of the adrenergic and serotonergic neurotransmitter systems in the brain: implications for differential drug effects on juveniles and adults. Biochem Pharmacol. 2007;73:1225–1236. doi: 10.1016/j.bcp.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman-Tancredi A, Rivet J, Chaput C, Touzard M, Verrièle L, Millan MJ. The 5HT1A receptor ligand, S15535, antagonises G-protein activation: a [35S]GTP[gamma]S and [3H]S15535 autoradiography study. Eur J Pharmacol. 1999;384:111–121. doi: 10.1016/s0014-2999(99)00491-4. [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus SC, Shaffer D. Antidepressant drug therapy and suicide in severely depressed children and adults: a case–control study. Arch Gen Psychiatry. 2006;63:865–872. doi: 10.1001/archpsyc.63.8.865. [DOI] [PubMed] [Google Scholar]
- Palmer RHC, Young SE, Hopfer CJ, Corley RP, Stallings MC, Crowley TJ, et al. Developmental epidemiology of drug use and abuse in adolescence and young adulthood: evidence of generalized risk. Drug Alcohol Depend. 2009;102:78–87. doi: 10.1016/j.drugalcdep.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MK, Belluzzi JD, Han S, Cao J, Leslie FM. Age, sex and early environment contribute to individual differences in nicotine/acetaldehyde-induced behavioral and endocrine responses in rats. Pharmacol Biochem Behav. 2007;86:297–305. doi: 10.1016/j.pbb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press: New York; 2007. [Google Scholar]
- Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Pradhan AAA, Cumming P, Clarke PBS. [125I]Epibatidine-labelled nicotinic receptors in the extended striatum and cerebral cortex: lack of association with serotonergic afferents. Brain Res. 2002;954:227–236. doi: 10.1016/s0006-8993(02)03340-1. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Ator R, Emmett-Oglesby MW, Hen R. Intravenous cocaine self-administration in mice lacking 5-HT1B receptors. Pharmacol Biochem Behav. 1997;57:407–412. doi: 10.1016/s0091-3057(96)00444-3. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self-administration of cocaine within the posterior ventral tegmental area of Wistar rats: evidence for involvement of serotonin-3 receptors and dopamine neurons. J Pharmacol Exp Therap. 2005;313:134–145. doi: 10.1124/jpet.104.075952. [DOI] [PubMed] [Google Scholar]
- Savitz J, Lucki I, Drevets WC. 5-HT(1A) receptor function in major depressive disorder. Progr Neurobiol. 2009;88:17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman E, Fallon S, Sershen H, Lajtha A. Nicotine-induced monoamine neurotransmitter changes in the brain of young rats. Brain Res Bull. 2008;76:626–639. doi: 10.1016/j.brainresbull.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Lê AD. Acute nicotine enhances c-fos mRNA expression differentially in reward-related substrates of adolescent and adult rat brain. Neurosci Lett. 2007;418:286–291. doi: 10.1016/j.neulet.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Rudder CL, Ryde IT, Tate CA, Seidler FJ. Permanent, sex-selective effects of prenatal or adolescent nicotine exposure, separately or sequentially, in rat brain regions: indices of cholinergic and serotonergic synaptic function, cell signaling, and neural cell number and size at 6 months of age. Neuropsychopharmacology. 2007;32:1082–1097. doi: 10.1038/sj.npp.1301231. [DOI] [PubMed] [Google Scholar]
- Solinas M, Scherma M, Fattore L, Stroik J, Wertheim C, Tanda G, et al. Nicotinic alpha 7 receptors as a new target for treatment of cannabis abuse. J Neurosci. 2007;27:5615–5620. doi: 10.1523/JNEUROSCI.0027-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, et al. Nicotine activation of {alpha}4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D1-like receptors in rat cortical and striatolimbic brain regions: An autoradiographic study. Dev Neurosci. 1999;21:43–49. doi: 10.1159/000017365. [DOI] [PubMed] [Google Scholar]
- Tassin J. Uncoupling between noradrenergic and serotonergic neurons as a molecular basis of stable changes in behavior induced by repeated drugs of abuse. Biochem Pharmacol. 2008;75:85–97. doi: 10.1016/j.bcp.2007.06.038. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torabi MR, Bailey WJ, Majd-Jabbari M. Cigarette smoking as a predictor of alcohol and other drug use by children and adolescents: evidence of the ‘gateway drug effect'. J School Health. 1993;63:302–306. doi: 10.1111/j.1746-1561.1993.tb06150.x. [DOI] [PubMed] [Google Scholar]
- Uchida S, Hotta H, Kawashima K. Long-term nicotine treatment reduces cerebral cortical vasodilation mediated by alpha4beta2-like nicotinic acetylcholine receptors in rats. Eur J Pharmacol. 2009;609:100–104. doi: 10.1016/j.ejphar.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Valjent E, Mitchell JM, Besson M, Caboche J, Maldonado R. Behavioural and biochemical evidence for interactions between delta 9-tetrahydrocannabinol and nicotine. Br J Pharmacol. 2002;135:564–578. doi: 10.1038/sj.bjp.0704479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. Into the world of illegal drug use: exposure opportunity and other mechanisms linking the use of alcohol, tobacco, marijuana, and cocaine. Am J Epidemiol. 2002;155:918–925. doi: 10.1093/aje/155.10.918. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Sofuoglu M. The impact of cigarette smoking on stimulant addiction. Am J Drug Alcohol Abuse. 2009;35:12–17. doi: 10.1080/00952990802326280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M, Reichenberg A, Grotto I, Yasvitzky R, Rabinowitz J, Lubin G, et al. Higher rates of cigarette smoking in male adolescents before the onset of schizophrenia: a historical-prospective cohort study. Am J Psychiatry. 2004;161:1219–1223. doi: 10.1176/appi.ajp.161.7.1219. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, et al. Misuse and diversion of stimulants prescribed for ADHD. J Am Acad Child Adolesc Psychiatry. 2008;47:21–31. doi: 10.1097/chi.0b013e31815a56f1. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Broide RS, Chen Y, Leslie FM. Highly sensitive radioactive in situ hybridization using full length hydrolyzed riboprobes to detect alpha 2 adrenoceptor subtype mRNAs in adult and developing rat brain. Brain Res Brain Res Protocols. 1999;3:229–241. doi: 10.1016/s1385-299x(98)00043-9. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Leslie FM. Codistribution of nicotinic acetylcholine receptor subunit α3 and α4 mRNAs during rat brain development. J Comp Neurol. 1997;386:540–554. doi: 10.1002/(sici)1096-9861(19971006)386:4<540::aid-cne2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Sidhpura N, Balfour DJ. Nicotine: from molecular mechanisms to behaviour. Curr Opin Pharmacol. 2005;5:53–59. doi: 10.1016/j.coph.2004.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.