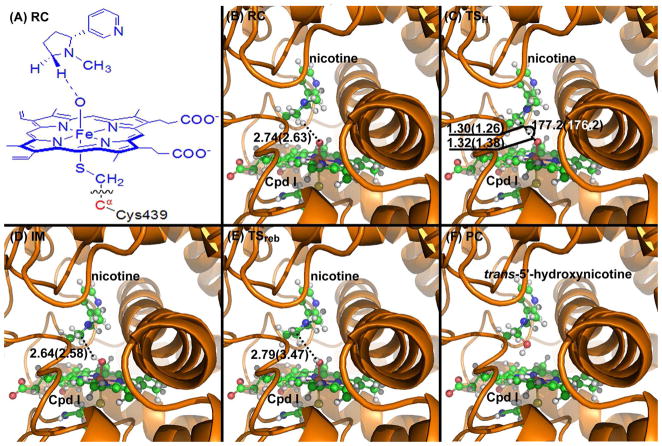
Figure 4.
(A) Division of the QM/MM system. Atoms in blue are treated by the QM method. The boundary carbon atom (colored in red) is treated with the improved pseudobond parameters. All other atoms belong to the MM subsystem. (B) to (F) Optimized geometries of key configurations of CYP2A6-catalyzed trans-5′-hydroxylation of (S)-(−)-nicotine. The geometries were optimized at the QM/MM (B3LYP/B1:AMBER) level. Nicotine and Cpd I are shown in ball-and-stick style, the rest of CYP2A6 is displayed in cartoon style. Values outside the parentheses are for the quartet state, while the values in parentheses are for the doublet state. RC: reactant complex; TS: transition state; IM: intermediate; PC: product complex. Distances are in angstrom (Å) and angles are in degrees.