Abstract
Background
Reductions in numbers of circulating progenitor cells (CD34+ cell subsets) have been demonstrated in patients at risk for, or in the presence of, cardiovascular disease. The mediators of these reductions remain undefined. To determine whether neurohumoral factors might regulate circulating CD34+ cell subsets in vivo, we studied complementary canine models of left ventricular (LV) dysfunction.
Methods and Results
A pacing model of severe LV dysfunction and a hypertensive renal wrap (RW) model in which dogs were randomized to receive deoxycorticosterone acetate (DOCA) were studied. Circulating CD34+ cell subsets including hematopoietic precursor cells (HPCs:CD34+/CD45dim/VEGFR2-) and endothelial progenitor cells (EPCs:CD34+/CD45-/VEGFR2+) were quantified. Additionally, the effect of mineralocorticoid excess on circulating progenitor cells in normal dogs was studied. The majority of circulating CD34+ cells expressed CD45 dimly and did not express VEGFR2, consistent with an HPC phenotype. HPCs were decreased in response to pacing, and this decrease correlated with plasma aldosterone levels (Spearman Rank correlation = -0.67, p=0.03). In the RW model, administration of DOCA resulted in decreased HPCs. No changes were seen in EPCs in either model. Normal dogs treated with DOCA exhibited a decrease in HPCs in peripheral blood but not bone marrow associated with decreased telomerase activity.
Conclusions
This is the first study to demonstrate that mineralocorticoid excess, either endogenous or exogenous, results in reduction in HPCs. These data suggest that mineralocorticoids may induce accelerated senescence of progenitor cells leading to their reduced survival and decline in numbers.
Keywords: heart failure, hypertension, progenitor cells, aldosterone
Introduction
There is growing evidence that bone marrow-derived progenitor cells may have reparative roles in cardiac and vascular disease and may serve as diagnostic and prognostic biomarkers.1, 2 Although the exact nature of these cells remains a topic of great interest, much of the work has focused on cells that are isolated by expression of CD34 or generated in vitro from circulating cells that express CD34.3 Cells originally identified in vitro as endothelial progenitor cells (EPCs:CD34+/CD45-/VEGFR2+) likely represent cells of hematopoietic origin that have assumed an endothelial phenotype including expression of VEGFR2.4, 5 These cells have been demonstrated to be capable of important functional effects likely due to paracrine mediators.4 Circulating CD34+ hematopoietic precursor cells (HPCs: CD34+/CD45dim/VEGFR2-) are distinct from EPCs by the lack of VEGFR2 expression and by the expression of CD45, and may be the source of so-called outgrowth endothelial cells in culture.6 We recently demonstrated in humans that rigorous characterization of CD34+ cell subsets provides distinct correlates of cardiovascular risk.7 In fact, it was the HPC rather than the circulating EPC population that was downregulated in patients with coronary endothelial dysfunction. This distinction may reflect the importance of hematopoietic precursors in the early stages of atherosclerosis. Although there is growing evidence that circulating progenitor cells may be regulated in heart failure (upregulated in Class 1 and downregulated in severe CHF),8 the goal of the current study was to utilize large animal models of cardiac dysfunction to assess the role of neurohumoral activation in the downregulation of circulating CD34+ cells.
Activation of the renin-angiotensin-aldosterone system (RAAS) occurs early in the pathophysiology of congestive cardiac failure,9 and the effects of this in the promotion of salt and water retention and the development of myocardial fibrosis are undisputed.9, 10 Serum aldosterone levels are potently associated with increased mortality in both systolic and diastolic heart failure11 whereas mineralocorticoid receptor blockade has beneficial effects in human and experimental heart failure and myocardial infarction.12, 13
It has recently been shown that aldosterone impairs progenitor cells in vitro.14 We, therefore, hypothesized that mineralocorticoid excess might be associated with reductions in circulating progenitor cell counts in animal models of cardiovascular disease and that this association may be mediated by reduction in production or survival of circulating progenitor cells. Thus, we studied distinct but complementary canine models of cardiovascular disease with or without endogenous and exogenous mineralocorticoid excess. Further, to understand whether mineralocorticoid excess affected abundance or survival of progenitor cells, peripheral and bone marrow progenitor cells were studied in normal dogs before and after exogenous administration of mineralocorticoid. To quantify progenitor cells in these models, we adapted established methodology from human studies7 and utilized canine-specific reagents to study hematopoietic (HPCs) and endothelial progenitor cells (EPCs).
Methods
Animal models
Established canine models of cardiovascular disease were utilized in this study. All animal studies were performed in accordance with the Animal Welfare Act and approved by the Mayo Clinic Institutional Animal Care and Use Committee.
Pacing model
The paced systolic dysfunction model is a well-studied model of dilated cardiomyopathy. Severe congestive cardiac failure was induced in 10 male mongrel dogs (weight: 20 to 30kg) by rapid right ventricular pacing at 240 bpm as described previously in detail.15 Blood was drawn at baseline and on the day of acute study in all animals. After 10 days of pacing, cardiorenal parameters were assessed in an acute study according to a protocol previously described.15, 16 Plasma aldosterone and atrial and B-type natriuretic peptide (ANP and BNP, respectively) were measured by radioimmunoassay.16
Progressive hypertensive heart disease with and without mineralocorticoid excess
Ten elderly dogs aged 7 to 10 years were studied as previously described.17 These dogs were the last 10 dogs studied in a recently published study.17 Briefly, all underwent a midline abdominal incision under general anesthesia with wrapping of both kidneys as previously described. All dogs were also instrumented with an indwelling intra-aortic catheter via the femoral artery for blood pressure measurement. After development of hypertension (5 weeks after renal wrap surgery), dogs were randomized to receive deoxycorticosterone acetate (1mg/kg/day for 3 weeks; RW DOCA) or no additional treatment (RW Control). As described in this model,17 DOCA increased conscious blood pressure and LV diastolic stiffness without a change in LV ejection fraction.
DOCA in normal dogs
An additional group of 6 young normal dogs underwent administration of DOCA at a similar dose of 1mg/kg/day IM for 10 days to ascertain a mechanism for the observations. Blood and bone marrow (by aspirate from the humeral head) were analyzed at baseline and at 10 days for CD34+ progenitor cell count. Blood pressures were measured at 10 days at the time of acute hemodynamic study.
Flow cytometry
Peripheral blood and bone marrow-derived cells were incubated with fluorochrome-conjugated antibodies to CD34-fluoroscein (R&D Systems), mineralocorticoid receptor (Abcam, Cambridge, MA), VEGFR2-APC (R&D Systems), and a biotinylated rat anti-canine CD45 antibody (R&D Systems) subsequently labeled with Streptavidin-PerCP (BD Biosciences). Murine IgG1 (R&D Systems) conjugated to Alexa 488, PE (Molecular Probes) and Rat anti-mouse PerCP (BD Biosciences) was used as isotype controls as well as IgG1-APC from Abd Serotec.
HPC (CD34+ CD45dim VEGFR2- cells) counts were analyzed using the ISHAGE single platform sequential gating strategy, which is an internationally validated standard18 (Supplemental Figure 1). EPCs (CD34+ CD45- VEGFR2+ cells) were enumerated by sequential gating on CD34+, CD45-, and VEGFR2+ cells (Supplemental Figure 2). Progenitor cell counts are expressed as % total leukocyte count or absolute cell counts as indicated. Demonstration of the anti-VEGFR2 antibody on canine aortic endothelial cells (Cell Applications, Inc.) is represented in Supplemental Figure 3.
Isolation of CD34+ cells from blood and bone marrow (uninstrumented normal dogs)
At baseline, 20ml of peripheral blood was drawn from the external jugular vein, and animals were anesthetized briefly with ketamine and diazepam followed by intubation and administration of an isoflurane/oxygen mixture via the endotracheal tube. The upper humeral area was shaved, prepped, and draped, and a 13-gauge bone marrow aspiration/biopsy needle (Medical Device Technologies, FL) was advanced through the distal end of the greater tubercle of the humerus at an angle of 45 degrees facing distally until the marrow cavity was entered (2-3mm). Ten ml of marrow was aspirated and transferred to an EDTA tube on ice. Animals were then allowed to recover, and DOCA administration was commenced 48 hours later. An identical blood draw was performed prior to the acute study. After induction and intubation, bone marrow aspiration was performed again as above prior to commencement of invasive hemodynamic assessment. Blood was processed by density gradient centrifugation with extraction of buffy coat as previously described.5, 7 Bone marrow was treated with 0.014% collagenase solution for 30 minutes, and the resulting cell suspension was diluted with PBS and layered over equal volumes of Ficoll-Paque PLUS™ (Amersham Biosciences, Uppsala, Sweden) for buffy coat extraction as with blood. Cells from blood or marrow were washed twice, counted, and Fc blocked with 1μg of mouse IgG per 105 cells. Approximately 7 million cells were kept for flow cytometry, which was performed as described above. The remainder (approximately 108 cells) was incubated with CD34-APC antibody (R&D systems) as per the manufacturer's instructions. Cells were washed twice and recounted and then incubated with Anti-APC Microbeads (Miltenyi Biotec, Germany) as specified by the manufacturer. Cells were again washed twice and resuspended in 50ml PBSFE for magnetic cell separation. Cells were separated using an AUTOMACS™ machine (Miltenyi Biotec) on a positive cell selection “posseld” program, collected, and pelleted for telomerase quantification as described below.
Quantification of telomerase activity by real time PCR
Telomerase activity was quantified using the real-time polymerase chain reaction-based Quantitative Telomerase Detection (QTD) Kit (Allied Biotech, Ijamsville, MD, USA) 19, 20 according to the manufacturer's protocol. Briefly, the CD34+ cells from the blood were collected, washed in PBS, and centrifuged for 30 minutes at 12 000×g at 4°C. The pellet was stored at -80°C until all samples were collected. All samples were run in triplicate. Positive (enclosed with the kit) and negative controls (heat inactivated product in lysis buffer) were included in the analysis. A standard curve for telomerase activity was generated using provided control templates. Telomerase activity is presented in relative units.
Statistical Analysis
Normally distributed data are reported as mean ± SEM. Non-normally distributed data are presented as median, [25th percentile, 75th percentile]. Within-group comparisons were performed using the Wilcoxon signed rank test. For between-group comparisons with repeated measures, regression analysis of post treatment counts as the y variable, treatment group as the x variable of interest, and mean pre-treatment count as a covariate, was undertaken. Simple associations between cell counts and neurohormonal markers were assessed by Spearman rank correlation. The level of significance was set at p<0.05
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
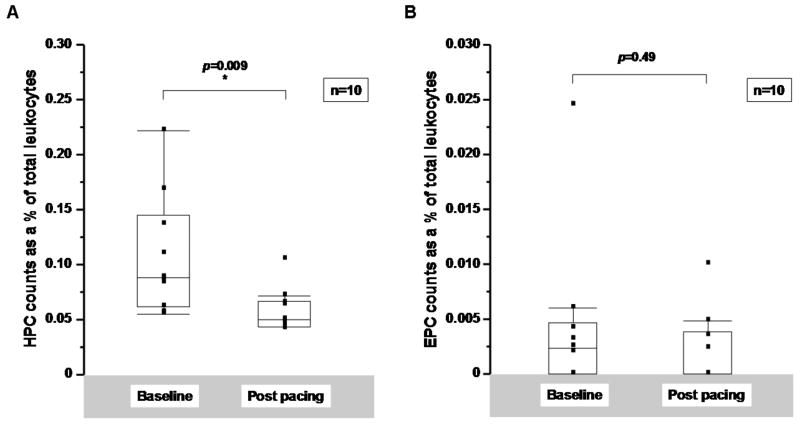
Rapid cardiac pacing resulted in hemodynamic evidence of advanced congestive cardiac failure as evidenced by decreased cardiac output (2.2, [1.8, 2.5] L/min) and increased systemic vascular resistance (40.4, [33.8, 51.7] WU) and pulmonary capillary wedge pressure (22.5, [14.8, 25.5] mmHg). In dogs, the majority of circulating CD34+ cells was CD45dim and VEGFR2- consistent with an HPC phenotype (95.8±1.9%). There was a significant decrease in HPCs (CD34+/CD45dim/VEGFR2-) after 10 days of pacing (0.09 [0.06, 0.15] % of circulating leukocytes at baseline versus 0.05 [0.04, 0.07] % at 10 days, p=0.009 by Wilcoxon signed rank test) (Figure 1A). No significant change in EPCs (CD34+/CD45-/VEGFR2+) was detected (0.002 [0, 0.005] % of circulating leukocytes at baseline versus 0 [0, 0.004] % at 10 days, p=0.49) (Figure 1B). Similar results were seen when absolute cell counts are compared (Supplemental Figure 3). There was no significant change in circulating leukocyte counts, measured as the number of total CD45+ cells per buffy coat isolate 3.4 [2.6,4.2] × 105 cells/100μL buffy coat at baseline versus 3.2 [2.2,3.7] × 105 cells/100μL buffy coat 10 days, p=0.57).
Figure 1.
A: Hematopoietic (HPC) and B: endothelial progenitor cell (EPC) counts in a canine pacing model of LV dysfunction. Cells were enumerated from paced dogs at baseline and at 10 days and compared using a Wilcoxon signed-rank test. Cell counts are expressed as a % of total leukocytes.
Neurohormonal parameters analyzed in the paced dogs at the time of acute hemodynamic study revealed a significant inverse correlation between the change in HPCs over the 10 day pacing period and the plasma aldosterone level (Spearman Rank Correlation =-0.67, p=0.03) (Table 1 and Supplemental Figure 4). No significant correlation was noted between changes in HPCs and serum ANP and BNP levels, nor did changes in EPCs correlate with plasma aldosterone, ANP, or BNP levels. These data suggest that elevation in serum aldosterone levels is associated, at least in part, with changes in circulating cell subsets.
Table 1.
Spearman Rank correlations between change in progenitor cell counts from paced dogs over 10 days compared with plasma aldosterone level, canine B-type natriuretic peptide (cBNP) levels, and canine atrial natriuretic peptide levels (ANP) at 10 days.
| Change in HPC count as a % of total leukocytes | Change in EPC count as a % of total leukocytes | |||
|---|---|---|---|---|
| Spearman rank correlation | p value | Spearman rank correlation | p value | |
| Plasma aldosterone level (ng/ml) | - 0.67 | *0.03 | 0.43 | 0.21 |
| Plasma cBNP level (pg/ml) | 0.10 | 0.78 | 0.24 | 0.49 |
| Plasma ANP level (pg/ml) | 0.15 | 0.70 | 0.19 | 0.61 |
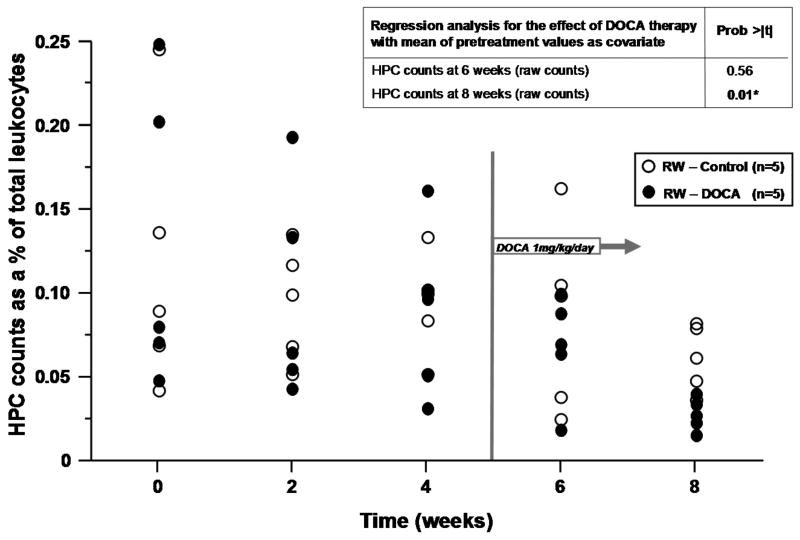
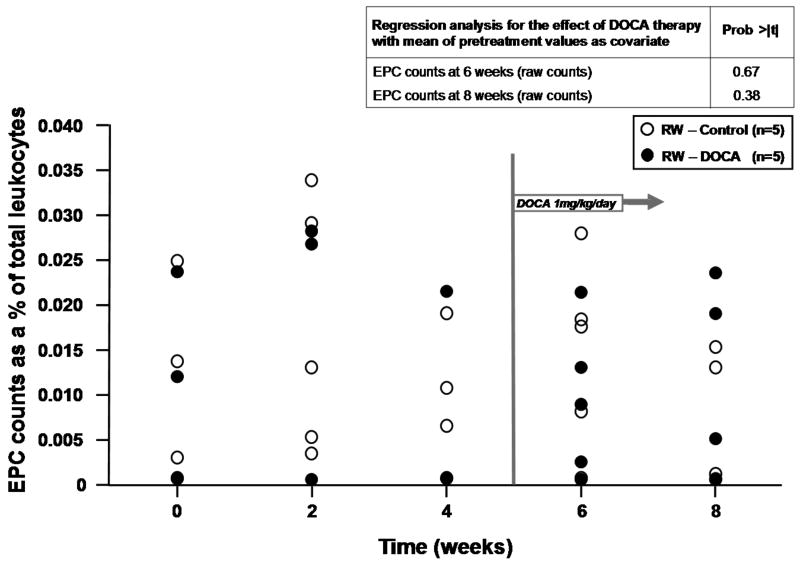
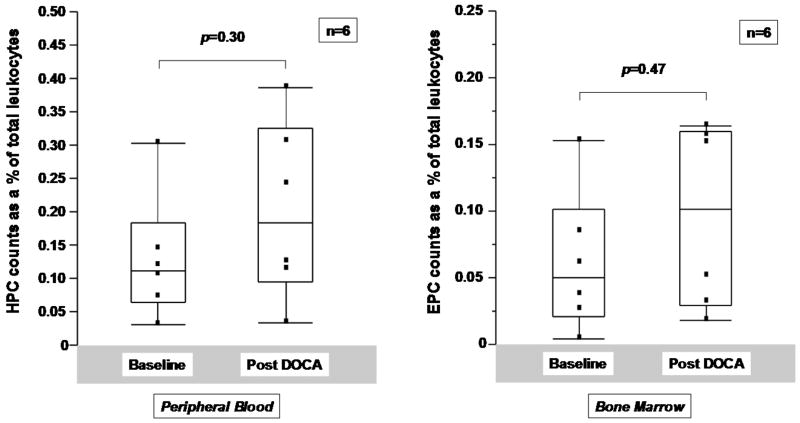
Based on the association between change in cell counts and aldosterone levels, we chose to further study the relationship between mineralocorticoid excess and CD34+ cell subsets in a model of hypertension which utilizes bilateral renal wrapping and administration of DOCA. DOCA has been shown to have important effects on left ventricular and vascular form and function in this model.17 The hemodynamic profiles of RW-control and RW-DOCA groups at the time of acute study are shown in Table 2. Again, the majority of circulating CD34+ cells was CD45dim and VEGFR2-consistent with an HPC phenotype (94.1±1.1%). When the effects of DOCA administration were analyzed at 6 and 8 weeks, regression analysis adjusting for pre-treatment mean as covariate revealed the differences in cell counts between groups at 8 weeks to be statistically significant (p=0.01) (Figure 2).
Table 2.
Hemodynamics at acute hemodynamic study in renal wrap groups (Median values shown).
| RW - Control n=5 |
RW - DOCA n=5 |
p value§ | |
|---|---|---|---|
| MAP* (mmHg) | 138.6 | 165.2 | 0.14 |
| PCWP† (mmHg) | 9.5 | 8.9 | 0.83 |
| CO† (l/min) | 2.0 | 1.8 | 0.53 |
| SVR† (WU) | 66.0 | 40.4 | 0.30 |
Conscious state
Under general anesthesia
Wilcoxon rank-sum test
Figure 2.
Effects of renal wrapping (RW) and deoxycorticosterone acetate (DOCA) on progenitor cell counts. Hematopoietic and endothelial progenitor cell counts over time with regression analysis for the effect of DOCA therapy with the mean of pretreatment values as covariate. (A) HPC and (B) EPC counts as % of total leukocytes.
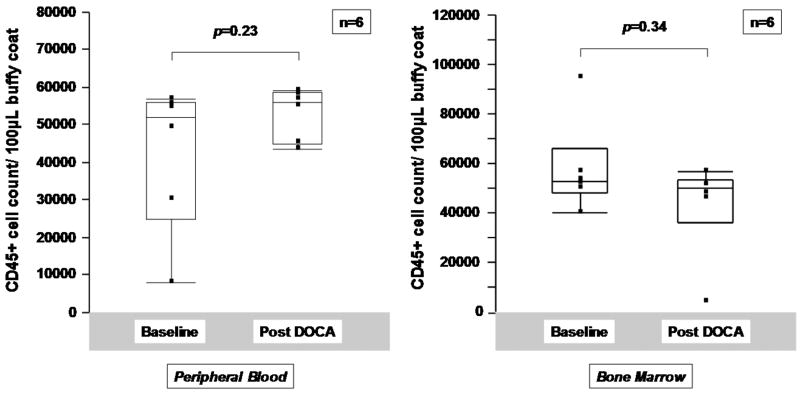
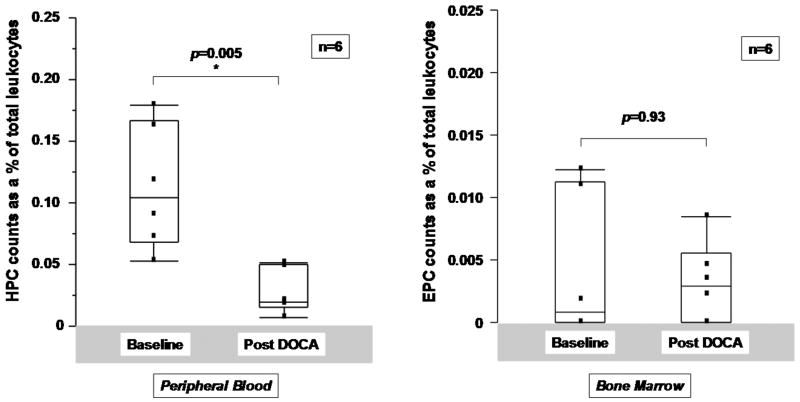
To determine whether mineralocorticoid excess is sufficient to account for decreases in HPCs, uninstrumented dogs were treated with DOCA for 10 days. No significant effect on total leukocyte counts was noted following DOCA treatment (Figure 3A). However, a significant fall in HPCs as a percentage of total leukocytes was noted in peripheral blood comparing baseline values with those at 10 days (0.10 [0.07, 0.17] % to 0.02 [0.02,0.05], p=0.005 by Wilcoxon signed rank test) (Figure 3B) but not in bone marrow (0.11 [0.06,0.18] % to 0.18 [0.09,0.33] %, p=0.30) (Figure 3C). EPCs did not change significantly in peripheral blood or in bone marrow following treatment with DOCA for 10 days (Figures 3B and 3C, rightward panels).
Figure 3.
Effects of DOCA administration on peripheral and bone marrow progenitor cells. A. DOCA administration did not affect peripheral blood or circulating CD45+ cell counts. B. HPC and EPC counts in buffy coat extracted from peripheral blood and C. from bone marrow in uninstrumented normal dogs pre and post 10 days of treatment with DOCA at a dose of 1mg/kg administered once daily intramuscularly. Analysis is by Wilcoxon signed rank test.
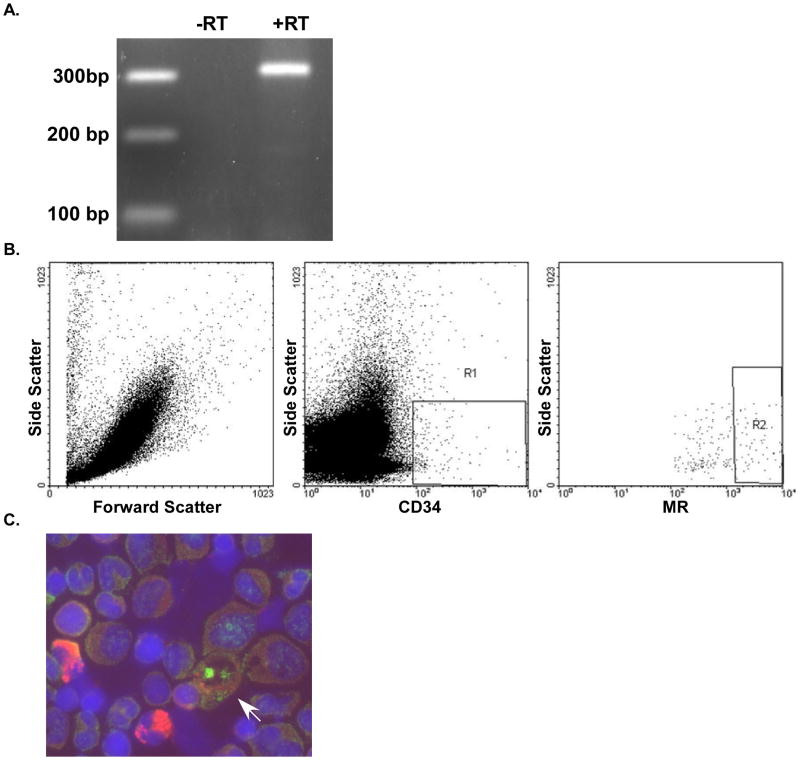
To determine whether cells in canine buffy coat express the mineralocorticoid receptor (MR), we demonstrated mRNA for MR present in these samples (Figure 4). Furthermore we performed FACS on canine blood and demonstrated co-expression of CD34 and MR. Immunostaining of canine bone marrow also showed co-expression of CD34 and MR.
Figure 4.
Detection of mineralocorticoid receptor (MR) expression. A. rt-PCR for MR in peripheral blood buffy coat from normal dogs. B. FACS of canine peripheral blood for CD34 and MR (R1=CD34+) (R2= CD34+/MR+). C. Co-localization of CD34 (red, cytoplasmic staining) and MR (green, nuclear staining) in canine bone marrow. Arrow indicates cell expressing CD34 and MR.
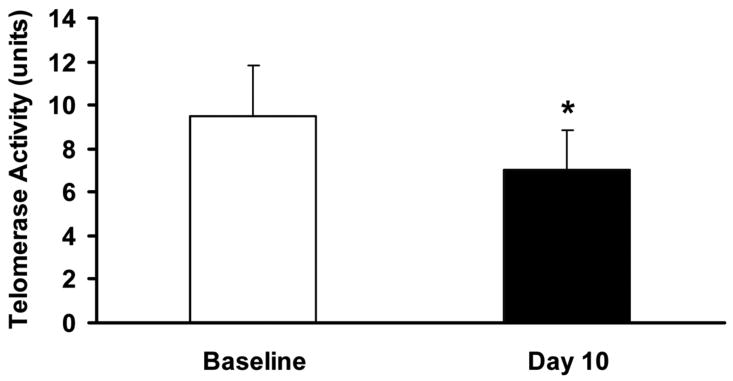
Finally, circulating CD34+ cells demonstrated evidence of senescence with reduced telomerase activity following administration of DOCA (Figure 5). No differences in telomerase activity were seen in bone marrow-derived CD34+ cells suggesting a peripheral effect. We were not able to perform these studies on CD34+ subsets. However, since the overwhelming majority of CD34+ cells are HPCs, we assume these findings represent this population. Taken together, DOCA may induce a decrease in circulating CD34+ cells through induction of senescence.
Figure 5.
DOCA decreases telomerase activity in CD34+ cells. Telomerase activity in lysed CD34+ cells extracted from peripheral blood at Baseline and following 10 days of DOCA treatment. *p=<0.05, Student t-test.
Discussion
It is now well established that progenitor cells of bone marrow origin can be detected in the circulation,3, 21, 22 and altered numbers and function of these cells have been noted in cardiovascular disease23-27 including heart failure.8 However, the mechanisms involved and the implications of these findings have remained elusive. In this study, two canine models of LV dysfunction were utilized. Specific canine reagents and analyses were used to quantify two populations of circulating CD34+ cells, EPCs, and HPCs. HPC counts were decreased in a paced model of cardiac failure. No changes in EPCs were noted. Interestingly, this decrease in HPCs correlated with increases in plasma aldosterone levels. Based on this original finding, we hypothesized that mineralocorticoid excess may be associated with decreased circulating HPCs. In this setting, aldosterone levels may be a surrogate for increased systemic oxidative stress. When DOCA, a mineralocorticoid receptor agonist was administered to the renal wrap model, there was a significant reduction in circulating HPC counts compared to controls. Again, no changes were seen in EPC counts. DOCA was also administered to normal dogs resulting in a similar reduction on HPCs observed after 10 days of treatment. In the latter animals, we also analyzed bone marrow pre- and post-treatment. It was interesting to note that the reduction in HPC number seen in the circulation was not seen in the bone marrow suggesting a peripheral effect on cell survival.
In this study, the majority of CD34+ cells expressed CD45dimly and did not express VEGFR2, consistent with the HPC phenotype. These HPCs were regulated by mineralocorticoid excess while no effects were seen in EPCs (CD34+/CD45-/VEGFR2+ cells). The lack of effect on EPCs may be due to their relative scarcity in the population, but their levels are consistent with our study in humans.7 It should be noted that the study was limited by a lack of canine specific reagents for other hematopoietic stem cell markers such as CD133, c-kit, or sca-1. Taken together, these data suggest that CD34+ cells are regulated in models of LV dysfunction associated with mineralocorticoid excess. However, it was the HPC population that accounted for these differences. As these HPCs may be the source of multiple relevant cell types including cells capable of assuming an endothelial phenotype, the findings have particular relevance to cardiovascular disease.
Our finding that CD34+ cells express the mineralocorticoid receptor is consistent with accumulating evidence that circulating progenitor cells express mineralocorticoid receptors28, 29 and also that their growth in vitro is impaired by addition of aldosterone to the culture medium.14 These findings are made all the more compelling by the observed reversal of these effects in vitro with the addition of spironolactone. These observations underscore the possibility that aldosterone exerts specific effects on circulating progenitor cells. However, the presence of the MR alone does not prove a direct effect. The expression of 11β-hydroxysteroid dehydrogenase is also a necessary component of MR activation in renal epithelial and vascular smooth muscle cells, and its expression in these cells is uncertain.30
Considered in this light, other biological mechanisms which may affect the number and function of these cells would include an increase in oxidative stress. It is undisputed that aldosterone is proinflammatory31, 32 and that it activates circulating mononuclear inflammatory cells.33, 34 It also exerts proinflammatory effects via central mechanisms including effects on sympathetic drive activity and production of TNFα.35 Accelerated senescence has also been attributed to aldosterone.36 High aldosterone levels in humans have been associated with reduced telomere counts in leukocytes.36 In addition, reduced telomere lengths have been seen in a spectrum of different cardiovascular disease states compared to controls.37 Large epidemiological trials have shown a clear correlation between telomere length and classical cardiovascular risk factors.38-40
Therefore, the possibility of increased senescence of circulating progenitor cells from mineralocorticoid treated animals was investigated as a mechanism leading to a decrease in their numbers. We found a significant difference in telomerase activity between CD34+ cells isolated from normal dogs at baseline and after 10 days of mineralocorticoid treatment. The reduction in telomerase activity seen in CD34+ cells after 10 days of DOCA therapy may reflect decreased protection from telomere damage ensuing proliferation of these cells which leads to apoptosis and a reduction in numbers. The result of this effect on the heart failure phenotype is still a matter of speculation but could include a reduced capacity for reparative paracrine mechanisms mediated by circulating progenitor cells.
Aldosterone antagonism has proven beneficial not just in chronic heart failure13 but also in heart failure post myocardial infarction.12 Aldosterone antagonism may also be of benefit in the treatment of endothelial dysfunction.41 The possibility of accelerated senescence contributing to these disease processes and the possibility of aldosterone as a mediator cannot be ignored in this light. Studies will need to define whether aldosterone antagonism might attenuate the reductions in circulating progenitor cells in these models.
In summary, for the first time, a reduction in circulating progenitor cells has been linked to changes in serum levels of mineralocorticoids. Additionally, it is suggested that the underlying mechanisms may include effects on telomerase activity in circulating progenitor cells leading to possible telomere length shortening and accelerated cell death. The implications of these findings suggest a novel paradigm in which to consider aldosterone antagonism in human disease.
Supplementary Material
Acknowledgments
Special thanks to Jim Tarara and the staff at the Mayo Clinic Flow Cytometric Core Facility for their assistance with sample processing for flow cytometric analysis. Also thanks to Gail J. Harty, Donna M. Meyer, Denise Heublein, Sharon Sandberg, Cheryl Mueske, Laurel Kleppe, and Tyra Witt for their technical assistance and to Megan E. Crouch for her assistance with preparation of the manuscript.
Sources of Funding: This study was funded by the National Institutes of Health (HL76611).
Footnotes
Disclosures: None.
References
- 1.Liao R, Pfister O, Jain M, Mouquet F. The bone marrow--cardiac axis of myocardial regeneration. Prog Cardiovasc Dis. 2007;50:18–30. doi: 10.1016/j.pcad.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wojakowski W, Kucia M, Kazmierski M, Ratajczak MZ, Tendera M. Circulating progenitor cells in stable coronary heart disease and acute coronary syndromes: relevant reparatory mechanism? Heart. 2008;94:27–33. doi: 10.1136/hrt.2006.103358. [DOI] [PubMed] [Google Scholar]
- 3.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 4.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 5.Gulati R, Jevremovic D, Peterson TE, Chatterjee S, Shah V, Vile RG, Simari RD. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;93:1023–1025. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 6.Timmermans F, Van Hauwermeiren F, De Smedt M, Raedt R, Plasschaert F, De Buyzere ML, Gillebert TC, Plum J, Vandekerckhove B. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol. 2007;27:1572–1579. doi: 10.1161/ATVBAHA.107.144972. [DOI] [PubMed] [Google Scholar]
- 7.Boilson BA, Kiernan TJ, Harbuzariu A, Nelson RE, Lerman A, Simari RD. Circulating CD34+ cell subsets in patients with coronary endothelial dysfunction. Nat Clin Pract Cardiovasc Med. 2008;5:489–496. doi: 10.1038/ncpcardio1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valgimigli M, Rigolin GM, Fucili A, Porta MD, Soukhomovskaia O, Malagutti P, Bugli AM, Bragotti LZ, Francolini G, Mauro E, Castoldi G, Ferrari R. CD34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation. 2004;110:1209–1212. doi: 10.1161/01.CIR.0000136813.89036.21. [DOI] [PubMed] [Google Scholar]
- 9.Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345:1689–1697. doi: 10.1056/NEJMra000050. [DOI] [PubMed] [Google Scholar]
- 10.Young MJ, Lam EY, Rickard AJ. Mineralocorticoid receptor activation and cardiac fibrosis. Clin Sci (Lond) 2007;112:467–475. doi: 10.1042/CS20060275. [DOI] [PubMed] [Google Scholar]
- 11.Guder G, Bauersachs J, Frantz S, Weismann D, Allolio B, Ertl G, Angermann CE, Stork S. Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation. 2007;115:1754–1761. doi: 10.1161/CIRCULATIONAHA.106.653964. [DOI] [PubMed] [Google Scholar]
- 12.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 13.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 14.Marumo T, Uchimura H, Hayashi M, Hishikawa K, Fujita T. Aldosterone impairs bone marrow-derived progenitor cell formation. Hypertension. 2006;48:490–496. doi: 10.1161/01.HYP.0000235681.25685.cf. [DOI] [PubMed] [Google Scholar]
- 15.Lisy O, Lainchbury JG, Leskinen H, Burnett JC., Jr Therapeutic actions of a new synthetic vasoactive and natriuretic peptide, dendroaspis natriuretic peptide, in experimental severe congestive heart failure. Hypertension. 2001;37:1089–1094. doi: 10.1161/01.hyp.37.4.1089. [DOI] [PubMed] [Google Scholar]
- 16.Boerrigter G, Costello-Boerrigter LC, Cataliotti A, Lapp H, Stasch JP, Burnett JC., Jr Targeting heme-oxidized soluble guanylate cyclase in experimental heart failure. Hypertension. 2007;49:1128–1133. doi: 10.1161/HYPERTENSIONAHA.106.083832. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro BP, Owan TE, Mohammed S, Kruger M, Linke WA, Burnett JC, Jr, Redfield MM. Mineralocorticoid signaling in transition to heart failure with normal ejection fraction. Hypertension. 2008;51:289–295. doi: 10.1161/HYPERTENSIONAHA.107.099010. [DOI] [PubMed] [Google Scholar]
- 18.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5:213–226. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 19.Schuller CE, Jankowski K, Mackenzie KL. Telomere length of cord blood-derived CD34(+) progenitors predicts erythroid proliferative potential. Leukemia. 2007;21:983–991. doi: 10.1038/sj.leu.2404631. [DOI] [PubMed] [Google Scholar]
- 20.Trulsson LM, Velin AK, Herder A, Soderkvist P, Ruter A, Smeds S. Telomerase activity in surgical specimens and fine-needle aspiration biopsies from hyperplastic and neoplastic human thyroid tissues. Am J Surg. 2003;186:83–88. doi: 10.1016/s0002-9610(03)00119-3. [DOI] [PubMed] [Google Scholar]
- 21.Stump MM, Jordan GL, Jr, Debakey ME, Halpert B. Endothelium Grown from Circulating Blood on Isolated Intravascular Dacron Hub. Am J Pathol. 1963;43:361–367. [PMC free article] [PubMed] [Google Scholar]
- 22.Gunsilius E, Duba HC, Petzer AL, Kahler CM, Grunewald K, Stockhammer G, Gabl C, Dirnhofer S, Clausen J, Gastl G. Evidence from a leukaemia model for maintenance of vascular endothelium by bone-marrow-derived endothelial cells. Lancet. 2000;355:1688–1691. doi: 10.1016/S0140-6736(00)02241-8. [DOI] [PubMed] [Google Scholar]
- 23.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt-Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 25.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 26.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 27.Werner N, Wassmann S, Ahlers P, Schiegl T, Kosiol S, Link A, Walenta K, Nickenig G. Endothelial progenitor cells correlate with endothelial function in patients with coronary artery disease. Basic Res Cardiol. 2007;102:565–571. doi: 10.1007/s00395-007-0680-1. [DOI] [PubMed] [Google Scholar]
- 28.Grafte-Faure S, Leveque C, Vasse M, Soria C, Norris V, Vannier JP. Effects of glucocorticoids and mineralocorticoids on proliferation and maturation of human peripheral blood stem cells. Am J Hematol. 1999;62:65–73. doi: 10.1002/(sici)1096-8652(199910)62:2<65::aid-ajh1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 29.Mirshahi M, Valamanesh F, Golestaneh N, Mirshahi P, Vincent L, Tang R, Agarwal MK. Mineralocorticoid hormones exert dramatic effects on pluripotent human stem cell progeny. Int J Toxicol. 2003;22:297–304. doi: 10.1080/10915810305118. [DOI] [PubMed] [Google Scholar]
- 30.Funder JW. Minireview: aldosterone and the cardiovascular system: genomic and nongenomic effects. Endocrinology. 2006;147:5564–5567. doi: 10.1210/en.2006-0826. [DOI] [PubMed] [Google Scholar]
- 31.Weber KT. A neuroendocrine-immune interface. The immunostimulatory state of aldosteronism. Herz. 2003;28:692–701. doi: 10.1007/s00059-003-2511-y. [DOI] [PubMed] [Google Scholar]
- 32.Weber KT, Gerling IC, Kiani MF, Guntaka RV, Sun Y, Ahokas RA, Postlethwaite AE, Warrington KJ. Aldosteronism in heart failure: a proinflammatory/fibrogenic cardiac phenotype. Search for biomarkers and potential drug targets. Curr Drug Targets. 2003;4:505–516. doi: 10.2174/1389450033490948. [DOI] [PubMed] [Google Scholar]
- 33.Gerling IC, Sun Y, Ahokas RA, Wodi LA, Bhattacharya SK, Warrington KJ, Postlethwaite AE, Weber KT. Aldosteronism: an immunostimulatory state precedes proinflammatory/fibrogenic cardiac phenotype. Am J Physiol Heart Circ Physiol. 2003;285:H813–821. doi: 10.1152/ajpheart.00113.2003. [DOI] [PubMed] [Google Scholar]
- 34.Ahokas RA, Warrington KJ, Gerling IC, Sun Y, Wodi LA, Herring PA, Lu L, Bhattacharya SK, Postlethwaite AE, Weber KT. Aldosteronism and peripheral blood mononuclear cell activation: a neuroendocrine-immune interface. Circ Res. 2003;93:e124–135. doi: 10.1161/01.RES.0000102404.81461.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lal A, Veinot JP, Leenen FH. Critical role of CNS effects of aldosterone in cardiac remodeling post-myocardial infarction in rats. Cardiovasc Res. 2004;64:437–447. doi: 10.1016/j.cardiores.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Benetos A, Gardner JP, Kimura M, Labat C, Nzietchueng R, Dousset B, Zannad F, Lacolley P, Aviv A. Aldosterone and telomere length in white blood cells. J Gerontol A Biol Sci Med Sci. 2005;60:1593–1596. doi: 10.1093/gerona/60.12.1593. [DOI] [PubMed] [Google Scholar]
- 37.Serrano AL, Andres V. Telomeres and cardiovascular disease: does size matter? Circ Res. 2004;94:575–584. doi: 10.1161/01.RES.0000122141.18795.9C. [DOI] [PubMed] [Google Scholar]
- 38.Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Samani NJ. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 39.Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, Kimura M, Larson MG, Meigs JB, Keaney JF, Aviv A. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 40.Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M, Aviv A. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 41.Imanishi T, Ikejima H, Tsujioka H, Kuroi A, Kobayashi K, Muragaki Y, Mochizuki S, Goto M, Yoshida K, Akasaka T. Addition of eplerenone to an angiotensin-converting enzyme inhibitor effectively improves nitric oxide bioavailability. Hypertension. 2008;51:734–741. doi: 10.1161/HYPERTENSIONAHA.107.104299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.