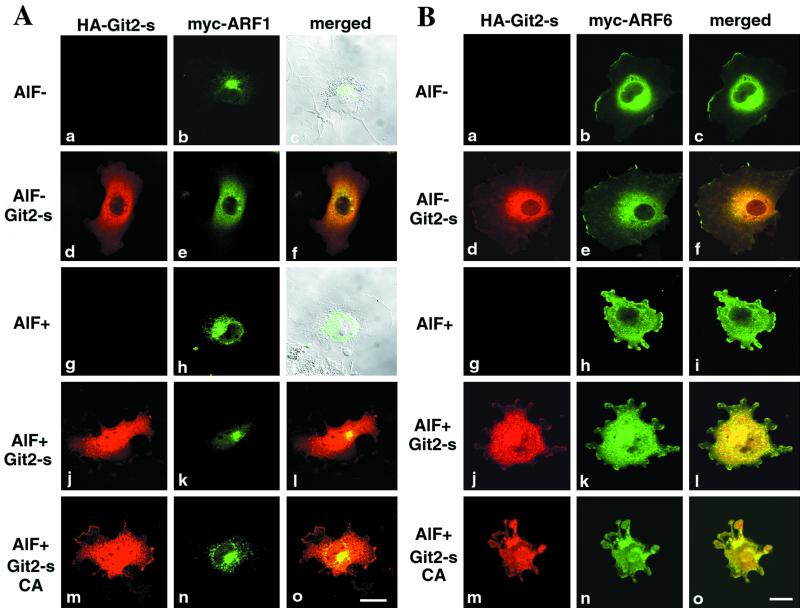
Figure 7.
Assessment of the ARFGAP activity of Git2-short in vivo. (A) Overexpression of Git2-short, but not its GAP-inactive CA mutant, suppresses the AlF-induced phenotype in ARF1 transfected cells. COS-7 cells were transiently transfected with a plasmid encoding myc-ARF1 alone (a–c and g–i) or a plasmid encoding myc-ARF1 was cotransfected with a plasmid encoding HA-Git2-short (d–f and j–l) or with the CA mutant of HA-Git2-short (m–o), using FuGENE 6. (B) Overexpression of Git2-short does not suppress the AlF-induced phenotype in ARF6 transfected cells. All panels correspond to those in A, except that a plasmid encoding myc-ARF6 was used instead of a plasmid encoding myc-ARF1. In A and B, all cDNAs were constructed in the pcDNA3 vector. For cotransfection, 0.5 μg of each Git2-short or the CA mutants and 0.5 μg of ARF plasmids were used, as determined by our preliminary titration experiments. Cells were untreated (a–c and d–f) or treated with AlF mixture for 1 h at 37°C (g–i, j–l, and m–o), before fixation. HA-Git2-short proteins were visualized by rabbit anti-HA antibody and Cy3 anti-rabbit IgG (d, j, and m). myc-ARF proteins were visualized with mouse anti-myc antibody coupled with Cy2 anti-mouse IgG (b, e, h, k, and n). The right columns represent the merging of the left and the middle images. Focuses were adjusted 3.0 μm above the surface of the plates. Bars, 20 μm. In c and i, differential interference contrast images (gray) are also included to show the subcellular localization of myc-ARF1. It should be noted that ARF1 existed as condensed structures localized to one side of the nucleus in the untreated COS-7 cells, as shown previously (Kondo et al., 2000); this is in contrast to the localization of ARF1 in 3Y1 cells, shown in Figure 3C.