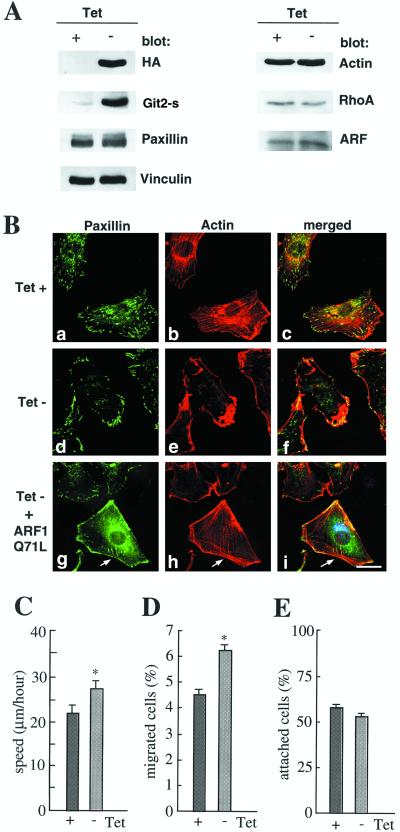
Figure 9.
Effects of Git2-short overexpression assessed in a single cell clone. An NIH3T3 cell clone bearing pTet-tTAK and pTet-Splice/HA-Git2-s was used. (A) Expression of HA-Git2-short, paxillin, vinculin, actin, Rho A, and ARFs in cells cultured in the presence (0.5 μg/ml, +) or in the absence (−) of tetracycline for 48 h. Cell lysates (20 μg) prepared with RIPA buffer (Sabe et al., 1994) were separated by SDS-PAGE (8%) and subjected to immunoblot analysis using antibodies as indicated. (B) Reduction in the number of paxillin-containing focal adhesion plaques and actin stress fibers by overexpression of HA-Git2-short and their partial restoration by co-overexpression of myc-ARFQ71L. Cells were cultured in the presence (a–c) or in the absence (d–i) of tetracycline, as above. Fibronectin-coated (10 μg/ml) chamber slides were used. a–c, d–f,and g–i are each of the same field; paxillin and F-actin were visualized as in Figure 8A. Arrows in g–i indicate a cell also expressing myc-ARF1Q71L (shown in i as blue), as visualized using rabbit anti-myc antibody and Cy2 anti-rabbit IgG. Focuses were adjusted 0.5 μm above the surface of the plates. Note that paxillin localization to focal complexes formed at the cell periphery was again almost unaffected or slightly augmented when HA-Git2-short was induced to be overexpressed (see d). Paxillin localization at the cell periphery was also augmented when ARF1Q71L was overexpressed (see g). (C–E) Effects of HA-Git2-short overexpression on cell migration on culture dishes (C), haptotaxis migration toward fibronectin in modified Boyden chambers (D), and cell adhesion to fibronectin (E). Cells were cultured in the presence (+) or in the absence (−) of tetracycline as above, and cell adhesion and migration activity were measured as described in MATERIALS AND METHODS. Each bar represents the mean ± SEM of triplicate experiments. *p < 0.02. In A–C, essentially the same results were obtained with three independent cell clones, in which HA-Git2-short was induced to be expressed at levels 20-fold higher than that of endogenous Git2-short. We also tried to examine the CA mutant overexpression in the same tetracycline-regulated system, but no cells overexpressed the CA mutant at levels similar to that of the wild-type HA-Git2-short clone. The cell clones we obtained could express the CA mutant up to about threefold higher than the endogenous Git2-short, and no significant changes in the amounts of paxillin-containing focal adhesions and stress fibers were observed (Mazaki and Sabe, unpublished results).