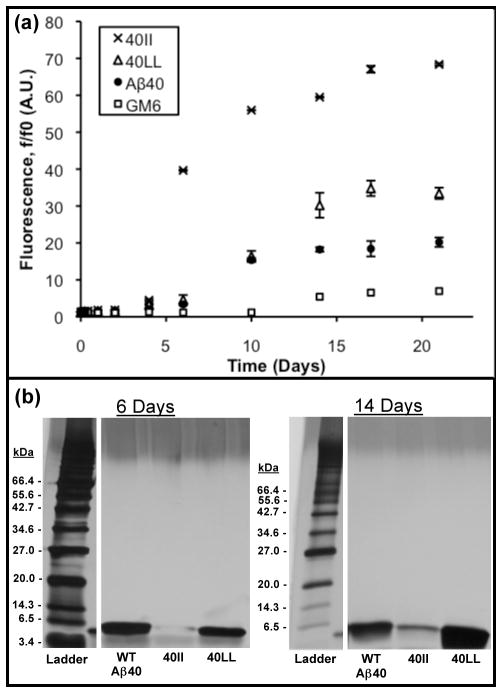
Figure 4.
(a) Time course of Thioflavin-T fluorescence showing increased amyloid formation by 40II and 40LL relative to WT-Aβ40. 20 μM solutions of peptide were incubated at 37°C under quiescent conditions. At the indicated time points, aliquots were mixed with ThT and measured at an emission wavelength of 483 nm. Time points are an average of three measurements and standard errors are indicated. (b) At 6 and 14 days, aliquots were centrifuged to remove insoluble aggregates, and boiled with SDS sample buffer before loading onto 10–20 % Tris-HCl gels. These gels confirm that 40II forms more insoluble aggregate (less monomer) than wild-type Aβ40. For 40LL, the quantity of monomer is comparable to WT Aβ40.