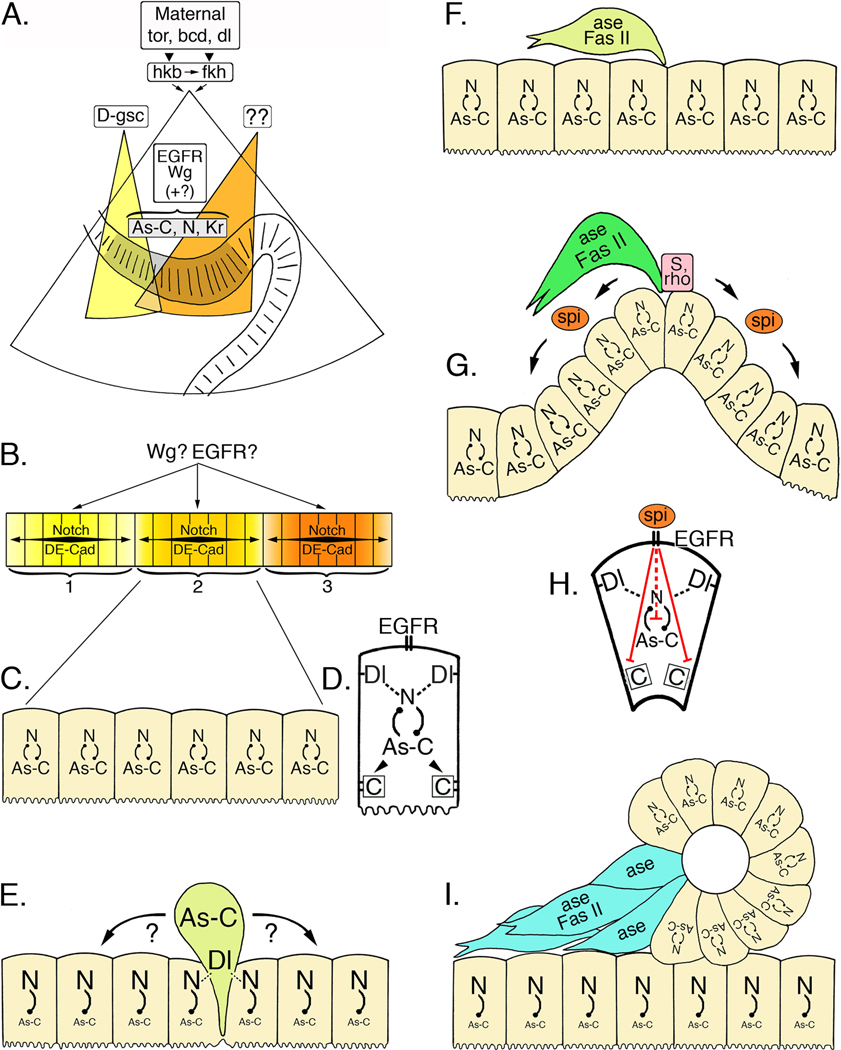
Figure 5. A cascade of regulatory genes controls neurogenesis in the ENS of Drosophila.
A. Maternally expressed torso (tor), bicoid (bcd), and dorsal (dl) control the expression of huckebein (hkb) and fork head (fkh) in the invaginating stomodeum (after Hartenstein, 1997). The homeobox gene D-goosecoid (D-gsc; yellow field) is required for the differentiation of the anterior stomodeum, including the anterior neurogenic center (zone 1) of the ENS. Other patterning genes (as yet unidentified; orange field) may specify the formation of the more posterior zones. As the stomodeum invaginates, the ENS anlage (grey shaded epithelium) becomes morphologically distinguishable and begins to express a combination of proneural genes in the Achaete-scute Complex (As-C), neurogenic genes (including Notch; N), and several other transcription factors, including Krüppel (Kr). Both Wingless (Wg) and EGFR signaling (plus other identified regulatory genes) play essential but poorly defined roles in this initial phase of ENS development. B. Wg and EGFR signaling may also help delineate the three neurogenic centers (1, 2, & 3) that subsequently form within the ENS anlage, possibly by limiting the range of Notch signaling within each zone and by modulating cell adhesive interactions mediated by Drosophila E-Cadherin (DE-Cad). C. All of the cells within each zone initially express intermediate levels of both proneural genes (As-C) and neurogenic genes (N). D. Enlarged view of a single zone cell at this stage. Notch (N)-Delta (Dl) interactions between adjacent cells are regulated in part by inhibitory feedback with the proneural genes (As-C); proneural genes may also regulate DE-Cadherin expression (boxed C). E. During the sequential delamination of individual precursors, lateral inhibition by the neurogenic genes promotes enhanced expression of the proneural genes (As-C) in a single zone cell, which then emerges from the foregut epithelium (light green cell represents a dSNSP). Cadherin-mediated adhesive interactions must also be down-regulated at this time. Concurrently, elevated levels of Notch signaling in the remaining zone cells help maintain their epithelial organization. F. Once delaminated, the precursor cell expresses the proneural gene Asense (ase), which may restrict further mitotic divisions, and the cell adhesion receptor Fas II, which may promote directed migration. The remaining zone cells re-acquire a balanced expression of both proneural genes and neurogenic genes. G. A second cycle of delamination gives rise to another set of precursors that emerge onto the foregut (dark green cell represents a tSNSP). As these precursors emerge, elevated levels of Star (S) and Rhomboid (rho) result in the localized release of the EGFR ligand, Spitz (spi), which in turn promotes the invagination of the remaining zone cells. H. Enlarged view of a single invaginating cell; EGFR signaling induced by Spitz interrupts the normal inhibitory feedback between neurogenic and proneural genes, permitting their continued co-expression. EGFR signaling also down-regulates DE-Cadherin-mediated adhesion, thereby promoting the morphological reorganization of the invaginating cells. I. Invagination of the neurogenic zone produces a discrete epithelial vesicle (see Fig. 3J), in which all of the cells continue to express both proneural genes (maintaining their potential to become neurons) and neurogenic genes (which help maintain their epithelial organization). By contrast, proneural gene expression is inhibited in the underlying epithelial layer. Individual cells from the vesicle then down-regulate neurogenic gene expression and disperse, while they upregulate Asense and Fas II (blue cells represent iSNSPs).