Abstract
Some of us (MAH) have known Dr. Eric Rose when he was a resident, a fellow in cardiothoracic surgery, the director of heart transplantation program, the director of Cardiothoracic Division, and finally as Valentine and Johnson and Johnson Professor and Chairman of Department of Surgery at Columbia University for the last 15 years. Having this long relationship with Dr. Rose, I was not sure where or how to begin this tribute to my former resident, colleague, collaborator, and eventually director. It was as an innovative and courageous Chairman that Dr. Rose had a major impact on me when he appointed me as a Residency Program Director and through his remarkable interest and support of educational changes led to a rebirth and growth of the surgical residency at Columbia NY Presbyterian Hospital to one of the leading programs in the country. But the greatest inspiration that Dr. Rose brought to the Department of Surgery was his fearless and relentless support of ventures into the unknown. When faced with heart transplantation as a junior faculty member, he went on to lay the foundation for the largest heart transplant program in the world; when challenged by development and lack of acceptance of Left Ventricular Assist Devices, he guided their approval following appropriate multiinstitutional studies which led to their adoption as standard of care. His influence extended to the support of a Pancreatic Islet Transplantation Program, originally inspired by his predecessor and mentor, Dr. Keith Reemtsma. Doctor Rose invested and encouraged both the clinical and experimental development of this program under my guidance as part of his dedication to innovation.
Following the remarkable early success of Edmonton’s group with steroid free protocol for clinical pancreatic islet transplantation, 10 other units, including ours, attempted to duplicate this effort with the support of NIH. With Dr. Rose’s support, and of colleagues in other departments, The Regional Columbia Pancreatic Islet Center joined the initial NIH Consortium (1). Our success was limited to only two successful clinical transplants, and this treatment lasted only 6 months. As we learned that others who transplanted more patients had limited success in having their recipients stay off insulin for more than 4 to 5 years (2) we returned to experimental work on development of a site for islet implantation other than intrahepatic to avoid early islet destruction by an inflammatory storm, poor engraftment, and possible intense exposure to higher toxic levels of immunosuppressive drugs in the liver. Our experiments focused again (3) on the intramuscular route of islet implantation, but this time using preconditioning of the recipients with three dimensional alginate scaffolds modified with vascular endothelial-derived growth factor (VEGF) and platelet-derived growth factor (PDGF) in combination to promote ingrowth of functionally sound vessels (4). In addition to these, we modified alginate with cyclic extracellular matrix signaling molecule arginine-glycine-aspartic acid peptide motif (RGD) which has been shown to enhance cellular adhesion and prevent transmembrane apoptotic signaling via integrins (5–7). The intent was to improve islet engraftment through avoidance low level of neovascularization. The choice of intramuscular site mimicked that of parathyroid autotransplantation and we felt was safer and more easily monitored without biopsies. It also offered the possibility of eventual local immunosuppression either with co-stimulatory blockade with monoclonal antibodies attached to the scaffold or with the use of immature dendritic cells co-transplanated with the islets (8–10).
The aims of the initial experiments was to separately study the efficacy of islet isografts with the use of scaffolds and on development of new methods of monitoring pancreatic islet survival using PET scanning techniques with available ligands. We then combined the experiments since such monitoring is best done intramuscularly and is not practical when the islets are in the liver because of the overwhelming background noise from the ligand.
The preliminary transplantation experiments using pancreatic islet isografts (2400 islets/recipient) into streptozotocin induced diabetic Lewis rat recipient primarily addressed the issues of neovascularization and engraftment at a intramuscular site. The islets were >90% pure. The relatively newer methods, published elsewhere (11–13) and modified by us, submitted elsewhere for publication (14), included priming of the alginate scaffolds with VGEF, PDGF, and with RGD prior to intramuscular implantation in the abdominal wall of each diabetic recipient of two scaffolds, either modified or unmodified (controls) 14 days before islet transplantation. This was to permit the area to be neovascularized before islet transplantation on day 0.
Detailed analysis of neovascularization at the transplant site just before islet implantation and at 2 weeks after their implantation with immunostaining revealed fibrovascular tissue penetrating scaffolds and a significantly higher number of capillaries stained with factor VIII related antigen per high power field in the scaffold containing VEGF/PDGF group compared to the other groups by 2–5 fold, p<0.05. Islet isograft survival for more than 2 months, as measured by normalized FBS and weekly GTT, was striking (100%) in the neovascularized groups preconditioned with modified scaffolds as compared to controls, some of which (20–40%) maintained improved, but not normalized blood glucose, since the islets were isografts and therefore were not rejected. When scaffolds were removed at 2 months, the animals reverted to being fully diabetic again. The details of these experiments, including the methodology, immunopathology, and results have been submitted for publication elsewhere (14)
Due to our expanding activities in clinical islet transplantation we, under the leadership of one of the co-authors (P.H.), also turned our attention to the issue of non-invasive monitoring of islet function after transplantation or after the onset of diabetes. Currently, islet allografts are monitored by metabolic measures which only detect graft dysfunction after substantial islet mass has already been lost (15). The non-invasive beta cell imaging technique that we recently developed relies on the use of PET with [11C] dihydrotetrabenazine (DTBZ) as means to quantitate vesicular monoamine transporter type 2 (VMAT2). VMAT2 is highly expressed relative to other cells in the beta cells of pancreas as it is located closely to insulin vesicles. This method cannot be applied to islets infused into the liver because liver and bile are routes of DTBZ excretion and have a high background signal. This approach originated by using gene profiling of purified human islets which grew out of our islet transplantation program (16).
Following islet transplantation to the intramuscular space, the PET scans with [11C] DTBZ demonstrated that the transplanted and viable beta cell mass can be visualized and quantified. The transplanted islets were visualized in animals that had gel+VEGF/PDGF/RGD modified scaffolds and that were normoglycemic, while those in the sham operated controls were not visible. It was interesting to also note that the native pancreas in STZ-treated animals had lower tracer binding that confirms that reversal of hyperglycemia was not due to residual beta cell mass in the native pancreas. This approach has now been used in humans and is detailed in previous publications (17, 18).
We have here briefly described the most recent experimental studies on islet transplantation performed in our laboratory, based on our unsuccessful attempts at long term or permanent reversal of diabetes mellitus in patients, where Dr. Rose played an important catalytic role. In line with his major interest in translational research, the studies that I have described have a great translational potential both for cellular transplantation and for development of a tool for diagnosis of disease progression or prevention in diabetes mellitus and of pancreatic islet allograft survival following transplantation.
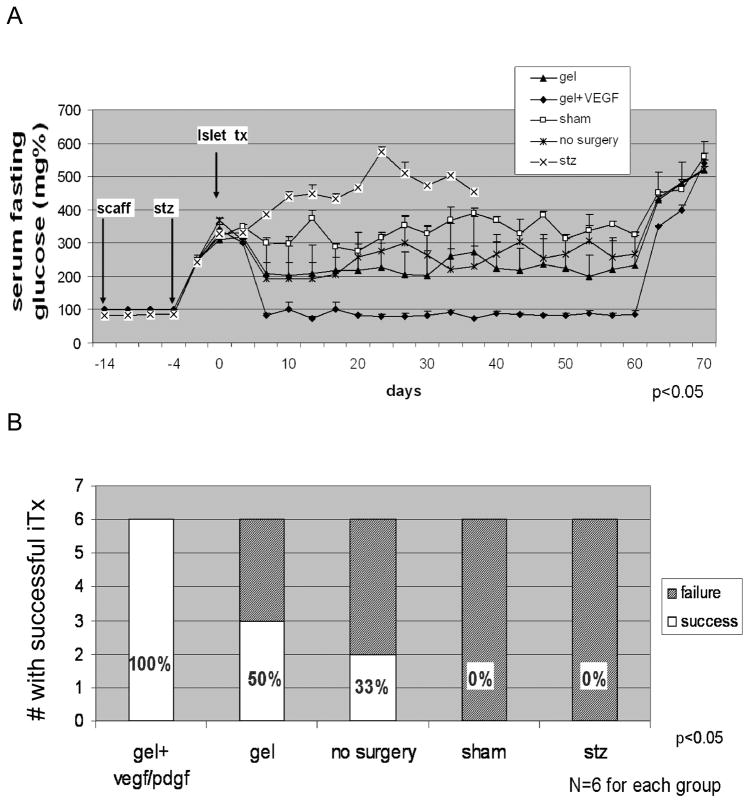
Figure 1. Blood Glucose Levels in Transplanted Rodents.
(A) Figure presents mean fasting serum glucose level in animals within the 60 days follow-up. Mean fasting glucose level oscillates below 110 mg/dL for animals from group 1 (gel + VEGF/PDGF), whereas in other groups it was statistically higher, ANOVA, p<0.05. As an additional control, removal of the scaffolds in normoglycemic animals from the gel + VEGF/PDGF group on day +60 led to prompt return of diabetes and hyperglycemia. Error bars represent S.E.M. (B) Islet grafting success rate. Number of animals out of six total achieving euglycemia following syngeneic islet transplantation is shown on the Y axis. Euglycemia was defined as four-hour fasting glucose < 110 mg/dL on post transplant days +5 through +60. The statistical significance of the differences in grafting success rate among the different treatment groups is shown and was determined by ANOVA using the blood glucose measurements obtained in the 55 day post transplant window.
Figure 2. Representative coronal plane of abdomen of Lewis Rats transplanted with allogeneic islets.
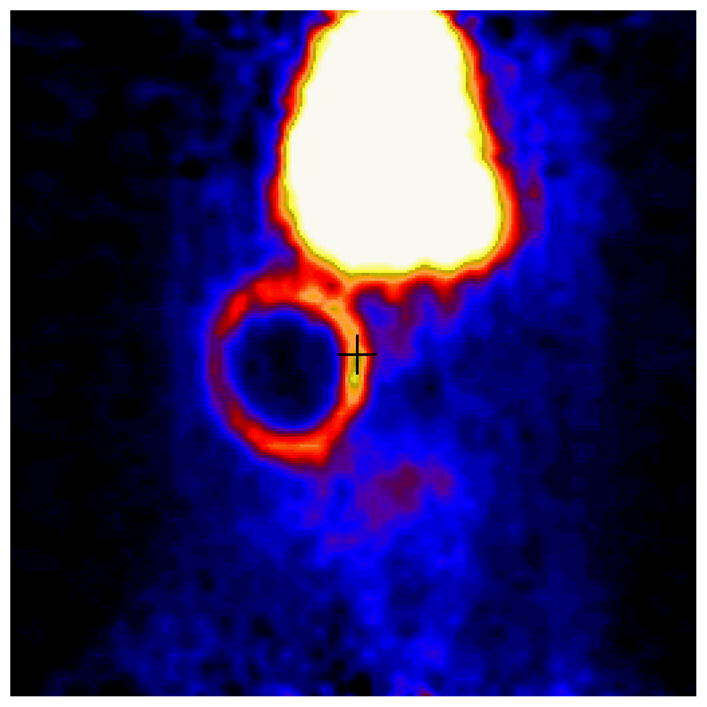
Rats were imaged (90 min) dynamically with 250 uCi [11C]DTBZ and a Concorde microPET scanner. The large high uptake area in the top center of the figure is a plane of the liver, an organ of [11C] DTBZ catabolism. Radioligand uptake in the form of a ring corresponds to the location of the transplanted islets. Following imaging, the presence of insulin staining cells in this location was confirmed by preparing paraffin embedded sections of the tissue and insulin staining by immunohistochemistry.
Acknowledgments
Supported in part by: NIH T32 HL 007854, NIH NIDDK 5RO1 DK63567-03
Supported in Part by:
NIH grant HL007854-12 (MAH)
References
- 1.Kaddis JS, Olack BJ, Sowinski J, Cravens J, Contreras JL, Niland JC. Human pancreatic islets and diabetes research. JAMA. 2009 Apr 15;301(15):1580–7. doi: 10.1001/jama.2009.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54 (7):2060. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 3.Weber CJ, Hardy MA, Pi-Sunyer F, Zimmerman E, Reemtsma K. Tissue culture preservation and intramuscular transplantation of pancreatic islets. Surgery. 1978;84 (1):166. [PubMed] [Google Scholar]
- 4.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19 (11):1029. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 5.Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24 (24):4385. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 6.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309 (5963):30. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 7.Wang RN, Rosenberg L. Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. J Endocrinol. 1999;163 (2):181. doi: 10.1677/joe.0.1630181. [DOI] [PubMed] [Google Scholar]
- 8.DePaz HA, Oluwole OO, Adeveri AO, Witkowski P, Jin MX, Hardy MA, Oluwole SF. Immature rat myeloid dendritic cells generated in low dose granulocyte macrophage–colony stimulating factor prolong donor-specific rat cardiac allograft survival. Transplantation. 2003;75:521–8. doi: 10.1097/01.TP.0000048380.84355.4A. [DOI] [PubMed] [Google Scholar]
- 9.Oluwole SF, Oluwole OO, DePaz HA, Adeyeri AO, Witkowski P, Hardy MA. CD4+CD25+regulatory T cells mediate acquired transplant tolerance. Transplant Immunology. 2003;11(3–4):287–93. doi: 10.1016/S0966-3274(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 10.Yang DF, Qiu WH, Zhu HF, Lei P, Wen X, Dai H, Zhou W, Shen GX. CTLA4-Ig-modified dendritic cells inhibit lymphocyte-mediated alloimmune responses and prolong the islet graft survival in mice. Transpl Immunol. 2008 Jul;19(3–4):197–201. doi: 10.1016/j.trim.2008.05.005. Epub 2008 Jun 17. [DOI] [PubMed] [Google Scholar]
- 11.Kedem A, Perets A, Gamlieli-Bonshtein I, Dvir-Ginzberg M, Mizrahi S, Cohen S. Vascular endothelial growth factor-releasing scaffolds enhance vascularization and engraftment of hepatocytes transplanted on liver lobes. Tissue Eng. 2005;11 (5–6):715. doi: 10.1089/ten.2005.11.715. [DOI] [PubMed] [Google Scholar]
- 12.Chen RR, Silva EA, Yuen WW, Mooney DJ. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm Res. 2007;24 (2):258. doi: 10.1007/s11095-006-9173-4. [DOI] [PubMed] [Google Scholar]
- 13.Pinkse GG, Bouwman WP, Jiawan-Lalai R, Terpstra OT, Bruijn JA, de Heer E. Integrin signaling via RGD peptides and anti-beta1 antibodies confers resistance to apoptosis in islets of Langerhans. Diabetes. 2006;55(2):312. doi: 10.2337/diabetes.55.02.06.db04-0195. [DOI] [PubMed] [Google Scholar]
- 14.Witkowski P, Hardy MA, et al. Transplanattion. (in press) [Google Scholar]
- 15.Shapiro AM, Hao EG, Lakey JR, et al. Novel approaches toward early diagnosis of islet allograft rejection. Transplantation. 2001;71 (12):1709. doi: 10.1097/00007890-200106270-00002. [DOI] [PubMed] [Google Scholar]
- 16.Maffei A, Liu Z, Witkowski P, Moschella F, Del Pozzo G, Liu E, Herold K, Winchester RJ, Hardy MA, Harris PE. Identification of tissue-restricted transcripts in human islets. Endocrinology. 2004 Oct;145(10):4513–21. doi: 10.1210/en.2004-0691. Epub 2004 Jul 1. [DOI] [PubMed] [Google Scholar]
- 17.Goland R, Freeby M, Parsey R, Saisho Y, Kumar D, Simpson N, et al. 11C-Dihydrotetrabenazine PET of the Pancreas in Subjects with Long-Standing Type 1 Diabetes and in Healthy Controls. J Nucl Med. 2009;50:382–9. doi: 10.2967/jnumed.108.054866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Souza F, Simpson N, Raffo A, Saxena C, Maffei A, Hardy M, Kilbourn M, Goland R, Leibel R, Mann JJ, Van Heertum R, Harris PE. Longitudinal noninvasive pet-based beta cell mass estimates in a spontaneous diabetes rat model. J Clin Invest. 2006;116:1506–13. doi: 10.1172/JCI27645. [DOI] [PMC free article] [PubMed] [Google Scholar]