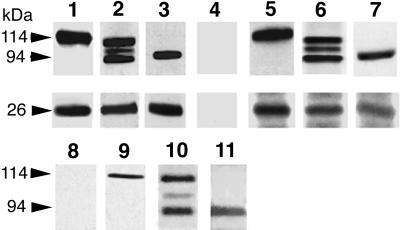
Figure 5.
Phocein and the proteins of the striatin family coimmunoprecipitate and pull down each other. Coimmunoprecipitation, lanes 1–7. Pansorbin cells, coated with preimmune immunoglobulins (lane 4) and affinity-purified anti-phocein antibodies (lanes 1–3) were incubated with rat brain cytosol. The washed pellets were analyzed by Western blots, using anti-striatin (lane 1, top), anti-SG2NA (lane 2, top), and anti-zinedin (lane 3, top) affinity-purified antibodies and anti-phocein purified antibodies (0.5 μg/ml; bottom part of the blot, lanes 1–3). Washed Pansorbin cells, coated with anti-striatin (lane 5), anti-SG2NA (lane 6), and anti-zinedin (lane 7) affinity-purified antibodies, were incubated with rat brain cytosol. The washed pellets were analyzed by Western blots. The blot was revealed by the anti-striatin serum (lane 5, top; no. 75, 1:2000), anti-SG2NA antibodies (0.2 μg/ml; lane 6, top), and anti-zinedin antibodies (lane 7, top; 1 μg/ml) and by affinity-purified anti-phocein antibodies (lanes 5–7, bottom). The ECL procedure was used. Pull-down experiments, lanes 8–11. Washed GST-coated beads (lane 8) and GST-phocein–coated beads (lanes 9–11) were incubated with brain cytosol and analyzed by Western blotting. The blots were revealed by an anti-striatin antiserum (no. 75, 1:2000; lane 9), affinity-purified anti-SG2NA (lane 10), anti-zinedin (lane 11) antibodies, and all three antibodies (lane 8). ECL procedure. The molecular masses of the proteins are indicated on the left (arrows).