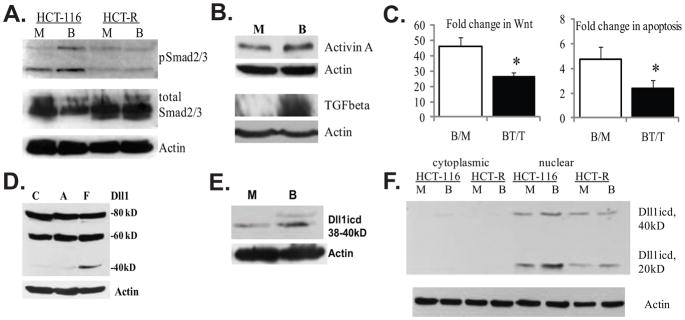
Fig. 1.
Evidence for TGFbeta/Activin signaling and expression of Dll1 species in HCT-116 colon cancer cells. (A). Butyrate induces the phosphorylation of Smad2/3 proteins in butyrate-sensitive HCT-116, but not in butyrate-resistant HCT-R cells. A representative Western blot of total and phosphorylated Smad2/3 in HCT-116 and HCT-R cells exposed to 24h of mock (M) or 5 mM sodium butyrate (B) treatment. The anti-phospho Smad2/3 antibody (sc-11769-R, Santa Cruz Biotechnology) recognizes the phosphorylated forms of both Smad2 and Smad3 (53kD and 60kD). (B). TGFbeta and Activin A expression is augmented in butyrate-treated HCT-116 cells. A representative Western blot analysis of Activin A expression in HCT-116 cells exposed to a 24-h mock (M) or 5 mM of sodium butyrate (B) treatment. Goat anti-Activin A antibody (R&D Systems, #AF338, used at 0.4 μg/ml), recognizes the Activin A dimer (28 kD), consisting of two inhibin beta-chains, as described by others [37]. (C) Suppression of TGFbeta/Activin signaling counteracts the induction of Wnt activity and apoptosis in butyrate-treated HCT-116 cells. Cells were transfected with the Wnt-sensitive luciferase reporters Lef-OT or Lef-OF, and the pRL-null vector, added as a measure for transfection efficiency. Transfected cells were exposed for 24h to mock treatment (M), 5mM butyrate (B), 20μM TGFbeta/Activin inhibitor SB-505124 from Santa Cruz Biotechnology (T), or 20μM inhibitor and 5mM butyrate (BT). Luciferase activity was assayed with dual luciferase kit (Promega), and the ratios of LefOT/LefOF, indicative of Wnt transcriptional activity were calculated. The B/M ratio is the fold increase of Wnt activity in butyrate- vs. mock-treated cells (625.0±72.7/13.9±3.49); whereas the BT/T ratio is the fold increase of Wnt activity in butyrate+inhibitor-treated cells versus inhibitor-treated cells (399.4±79.2/15.9±4.56), P<0.05. The data are the mean of three independent experiments with duplicate samples in each experiment. Apoptosis was measured in cells exposed to the same treatments (see Materials and Methods). The ratio B/M is the ratio of the percentages apoptotic cells in butyrate- vs. mock-treated samples (4.74±1.02); the ratio BT/T is the ratio of the percentages apoptotic cells in butyrate+inhibitor- treated samples vs. inhibitor-treated samples 2.41±0.62, P<0.05. We analyzed three samples per treatment in each of three independent experiments. Bars, SDs. (D). Highest levels of a Dll1icd-containing species are detected in floating fractions of butyrate-treated HCT-116 cells. Exposure of HCT-116 cells to 5 mM sodium butyrate for 48h results in a fraction of floating cells (F) and a fraction of substrate-adherent cells (A). Total protein lysates (150 μg) from these two cell fractions and mock-treated cells (C) were analyzed by Western blotting. The anti-Dll1 antibody detects the full length ligand (80kD), a form of the ligand with partially shed ectodomain (60kD), and a carboxy-terminal form of 38–40kD. (E) Butyrate-treated HCT-116 cells express higher levels of carboxy-terminal 38–40kD Dll1 species compared to mock-treated cells. A representative Western blot analysis of nuclear lysates (80 μg) of HCT-116 cells exposed to mock or 5 mM sodium butyrate treatment for 17h. Detection was achieved with a goat anti-Dll1 antibody (sc-8155, Santa Cruz Biotechnology) and a beta-Actin antibody (Sigma). (F) Highest levels of Dll1icd (20kD) are detected in nuclear fractions of butyrate-treated HCT-116 cells. Cytoplasmic-nuclear fractionation of HCT-116 cells exposed to mock (M) or 5 mM butyrate (B) treatment for 17h was performed with the Pierce Nuclear and Cytoplasmic Extraction Reagent Kit (NE-PER). A total of 100 μg of cytoplasmic or nuclear proteins were analyzed on 12% SDS gels. Western blot detection of Dll1 species was carried out with a rabbit anti-Dll1 antibody (sc-9102, Santa Cruz Biotechnology).