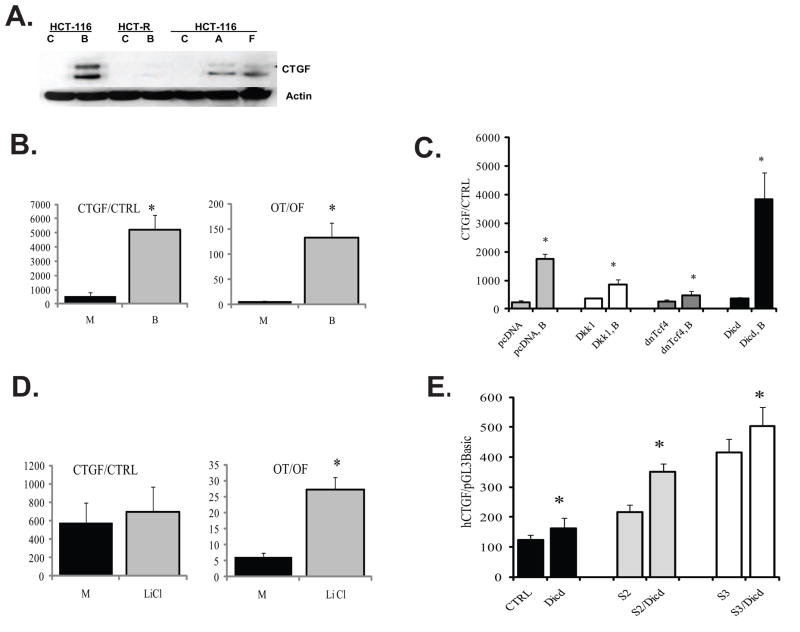
Fig. 4.
Butyrate induces CTGF expression in HCT-116 cells, and this effect is modulated by components of Wnt signaling and Dll1icd. (A) CTGF expression is induced by butyrate in butyrate-sensitive HCT-116 cells, but not in butyrate-resistant HCT-R cells. HCT-116 and HCT-R cells were exposed to mock (C) or 5 mM butyrate (B) treatment for 24h. In addition, HCT-116 cells were treated for 48h with 5 mM butyrate, and lysates from the floating (F) and substrate-adherent (A) cells were analyzed along with lysates of mock-treated (C) cells. Total protein lysates (100μg) were analyzed by Western blot analyses, CTGF and beta-Actin were detected with antibodies from Santa Cruz Biotechnology (sc-14939) and Sigma (A5441), respectively. The production of two CTGF isoforms of 35 and 38 kD, and smaller proteolytically processed forms have been previously reported [48]. (B) Butyrate treatment of HCT-116 cells upregulates the activity of the CTGF promoter. HCT-116 cells were co-transfected with the CTGF reporter and the normalization plasmid pRL-TK, or with the promoterless pGL3Basic and pRL-TK, in a 96-well plate format, following the Lipofectamine 2000 quick transfection protocol (see Materials and Methods). Cells were assayed for luciferase activity after 17-h exposure to mock (M) or 5 mM butyrate (B) treatment. For comparative analyses, the same transfection protocol was applied for Wnt signaling reporters (Lef-OT and Lef-OF). Each transfection was performed in duplicate wells; data represent the mean from results of at least three experiments. (C) Exogenous expression of Dll1icd, dnTcf4, and Dkk1 modulate the induction of CTGF by butyrate. HCT-116 cells were co-transfected with the CTGF promoter reporter (CTGF) or with its promoterless version (CTRL) and one of the following plasmids: pcDNA3Neo (empty vector), Dkk1-expression vector, dnTcf4-expression vector, or Dll1icd (Dicd)-expression vector. All transfections included pRL-TK as a control for transfection efficiency. We applied the quick transfection protocol for Lipofectamine 2000 in 96-well plates. Approximately 60,000 cells were transfected with reporter to effector plasmid ratio at 1:3, to a total of 320ng DNA per well and 0.8 μl of Lipofectamine. After six hours of incubation with the transfection mixture, cells were exposed to mock or 5 mM butyrate treatment for 17h. The ratio of CTGF promoter activity to that of the promoterless reporter was calculated for cells in absence or presence of butyrate. Data represent the mean from results of at least three transfections; each transfection was performed in duplicate wells. There were statistically significant differences between the CTGF promoter activities in butyrate-treated cells expressing Dkk1, dnTcf4, or Dicd and the CTGF promoter activity in butyrate-treated cells transfected with an empty pcDNA vector, (P-values were 0.001, 0, and 0.021, respectively). (D) Increase in active (dephosphorylated) beta-catenin is not sufficient for the induction of CTGF by butyrate. HCT-116 cells were transfected with CTGF or Wnt reporters as described in (B) and exposed to 20mM lithium chloride (LiCl) for 17h. Each transfection was performed in duplicate wells; data represent the mean from results of at least three experiments. There was no statistically significant difference between the activities of the CTGF promoter in mock- and LiCl-treated cells (P>0.05); however, control transfection experiments confirmed the ability of LiCl to induce the Wnt-sensitive promoter Lef-OT (P<0.05). (E) Co-expression of Dll1icd (Dicd) with constitutively active (ca) Smad2 (S2) or constitutively active Smad3 (S3) activates the CTGF promoter in the absence of butyrate. HCT-116 cells were co-transfected with the CTGF promoter reporter (CTGF) or the control pGL3Basic, and one of the following combinations of plasmids: pcDNA3Neo (empty vector), Dll1icd (Dicd)-expression vector, Dll1icd and caSmad2, or Dll1icd and caSmad3. We utilized the reverse Lipofectamine protocol in a 96-well format; the reporters were used at 80 ng per well, the effectors were used at total of 240 ng. pRL-TK was co-transfected at 1:200 ratio for transfection efficiency. Fresh medium was added at five hours post-transfection, and luciferase assays were performed at 24h post-transfection. Data represent the mean from results of at least three transfections; each transfection was performed in duplicate wells. Bars are SDs; statistically significant differences are marked with asterisks.