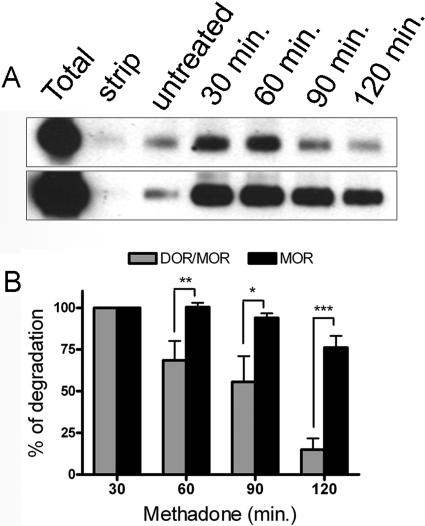
Fig. 7.
Endocytosis of the DOR/MOR heteromer leads to the degradation of the receptor complex. Postendocytic stability of DOR/MOR heteromers and MOR homomers from the same cell line were analyzed by biotin protection-degradation assay. Cells coexpressing FLAG-MOR and HA-DOR were biotinylated, then left untreated or treated with 1 μM methadone for 30, 60, 90, and 120 min before stripping. Total refers to the biotinylated receptor signal present in cells after initial labeling and without further manipulation; strip refers to biotinylated cells that were reacted with glutathione without other manipulations, demonstrating the efficiency with which biotin can be cleaved from surface receptors and represents the background. The stability of the protected endocytosed DOR/MOR heteromers (top) and MOR homomers (bottom) was assessed by serial immunoprecipitation followed by SDS-PAGE and streptavidin overlay (see Materials and Methods) at the time points stated. B, quantification of experiments in A is shown for DOR/MOR heteromers versus MOR homomers. Histogram shows the mean stability of the biotinylated endocytosed receptors relative to the endocytosed pool seen after 30 min of stimulation. Shown are the mean ± S.E.M. n = 4 to 10 independent experiments (two-way ANOVA, Bonferroni post-test: *, p < 0.05; **, p < 0.01; ***, p < 0.001).