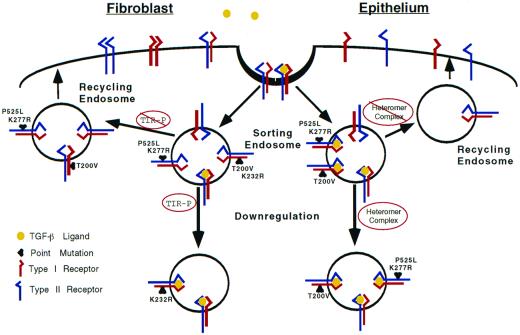
Figure 6.
Schematic diagram illustrating potential endocytic routes for heteromeric TGF-β receptor complexes in fibroblasts and epithelial cells. We propose this working model for the trafficking of TGF-β receptor complexes for the two cell types studied. Although ligand is included, it is presently unknown whether it remains receptor bound or dissociates from the receptor complex. Initially, receptors are internalized into a vesicular compartment (sorting endosome), which separates heteromeric and homomeric receptor complexes regardless of cell type (for simplicity only heteromeric complexes are shown). In the absence of ligand, heteromeric receptors are recycled back to the cell surface. A key feature for mesenchymal cells is the phosphorylation status of the type I receptor. Type I receptor phosphorylation is decreased by the P525L or K277R mutations in the type II receptor or the type I receptor T200V mutation; any of these receptor mutations will keep the receptor complex within the recycling pathway. However, if the type I receptor is phosphorylated in fibroblasts, the complex is diverted from the default recycling pathway to one associated with receptor down-regulation. This is in contrast to epithelial cells, which shuttle ligand-bound heteromeric receptor complexes to the down-regulation pathway(s) regardless of phosphorylation status.