Fig. 8.
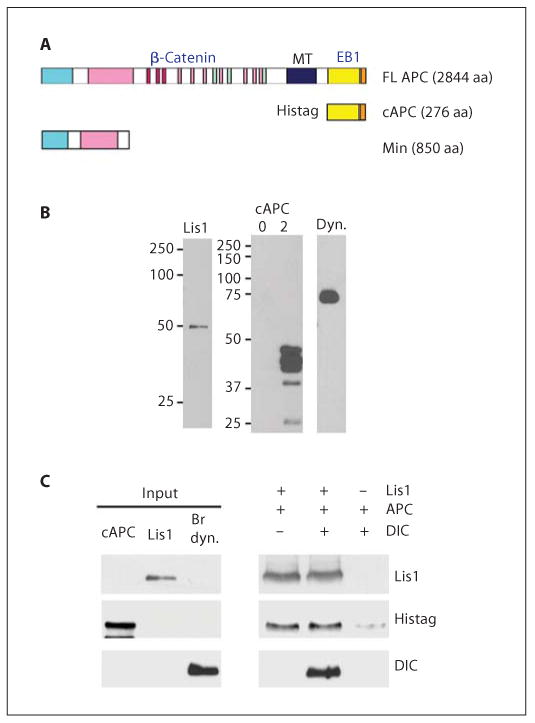
The C terminus of APC binds to purified Lis1 and dynein in vitro. A Schematic of APC forms showing protein interaction domains: FL APC = full-length APC; cAPC = histidine-tagged, C-terminal domain (aa 2577–2844); Min = N-terminal domain expressed in Min mice (aa 1–850); MT = microtubule-interacting domain. B Western blots of proteins used in these studies: left panel = Lis1, expressed in insect cells and purified by nickel chromatography is recognized by a Lis1 antibody; right panel = cAPC expressed in bacteria induced with isopropyl-β-d-thiogalactopyranoside for 2 h was labeled with a Histag antibody. No cAPC was detected in uninduced extracts (0 h, lane 1). GSTEB1 produced in bacteria is labeled with an EB1 antibody (lane 3). Dynein (dyn.) purified from bovine brains is labeled with a DIC antibody. C Lis1 at 10 pmol was added to 500 μl of the 2-hour cAPC extracts, in the presence or absence of 6 pmol dynein. Lis1 was immunoprecipitated. Coprecipitating proteins were analyzed by Western blotting. cAPC coprecipitates with Lis1, independent of dynein.
