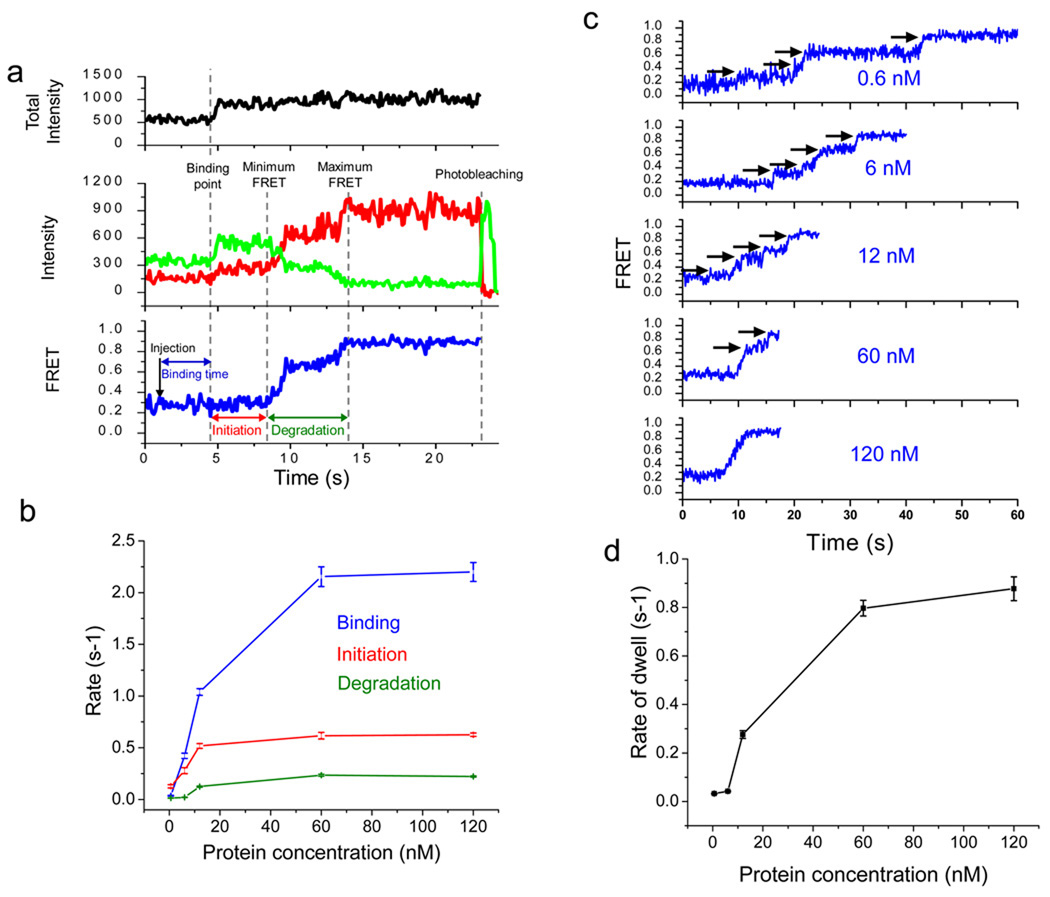
Figure 2.
λ exo carries out initiation and distributive degradation until it is stably engaged by the substrate. (a) A schematic illustration showing how binding time, initiation time and degradation time are assigned: (1) the protein binding period that begins when the reaction buffer is injected and ends right before the enhancement of both fluorescence intensities, (2) the initiation period during which fluorescence intensities increase but FRET stays at a constant low level, and (3) the degradation period during which FRET increases from the minimum to the maximum values. Total intensity is the sum of the donor and acceptor intensities (black in upper panel). Green and red curves represent the donor and acceptor intensities, respectively (middle panel), and the blue curve represents the calculated FRET efficiency (lower panel). (b) Inverse of characteristic times of binding, initiation and degradation vs. protein concentration. Each data point is an average of more than 200 molecules (see Supplementary Fig. 2–4 for their distributions). (c) Representative single molecule time traces for various protein concentrations. Below 60 nM protein, the time traces show multiple pauses, indicating frequent dissociation of λ exo from the substrate. (d) Average pause duration vs. protein concentration (see Supplementary Fig. 5 for their distributions). Error bars denote standard errors.