Fig. (1).
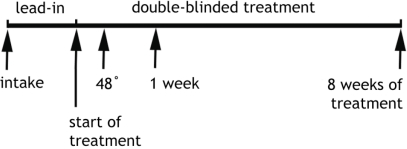
Experimental Protocol Timeline. Subjects were assessed and enrolled at “intake”, had the pretreatment baseline EEG recorded at that time, and then participated in a one week, single-blind placebo lead-in phase. Randomization to treatment modality (active medication or placebo) took place at the time marked “start of treatment”, Another EEG was recorded after 48 hours of treatment and again at 1 week. Clinical assessment to determine outcome (responder vs non-responder) took place after 8 weeks of treatment (9 weeks in study altogether). Subjects were monitored weekly during the double-blind treatment (arrows not shown) for clinical changes and adverse reactions [10].