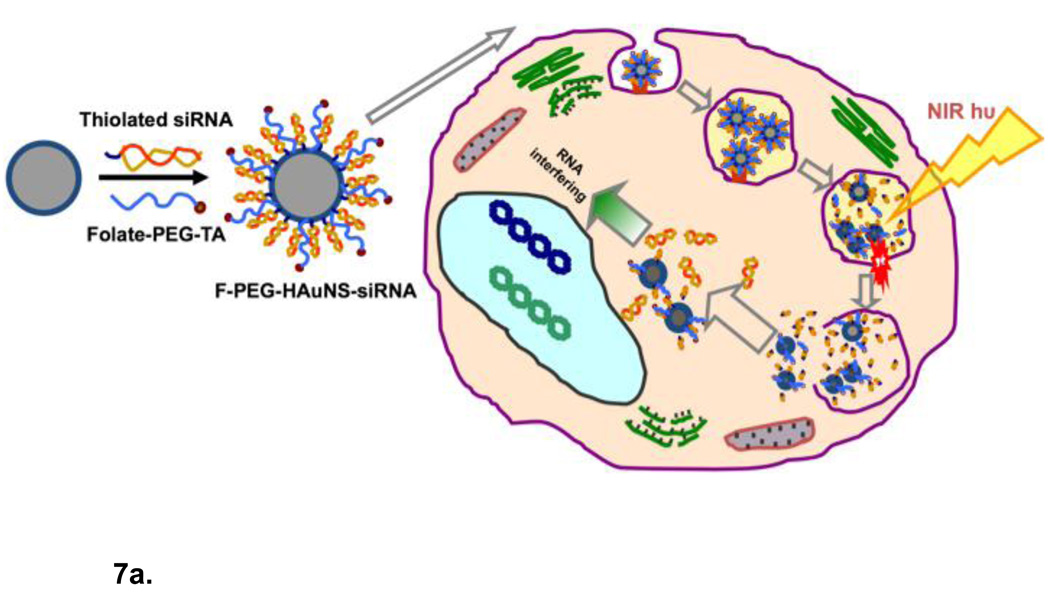
Figure 7.
(a) Schematic diagram of siRNA delivery vector based on hollow gold nanoshells and disrupting endosome membranes in living cells. Thiolated siRNA is coupled to the HGN to compact and protect the siRNA from degradation and deliver thousands of oligonucleotides with each nanoparticle. Thiolated PEG with targeting peptides can be used to stabilize the HGN against aggregation and target specific internalizing receptors on cells. Once the HGN-siRNA conjugate is internalized within a cell, usually within an endosome, pulsed NIR light of the appropriate intensity can be used to release the siRNA by first cleaving the thiol bonds to the gold, followed by a higher intensity pulse to disrupt the endosome membrane in the same way liposome membranes are disrupted though the formation of microbubbles (Fig. 6). The intact siRNA is released to the cell cytoplasm which allows for siRNA silencing of protein function. (b) Transmission electron micrographs of intracellular distribution of folate-labelled gold nanoshells, thiol linked to siRNA, without (top) or with (bottom) NIR pulsed laser irradiation. Arrow in the top left and top right panels indicate that prior to irradiation the HGN remain attached to the rim of endocytic vesicles. Arrows in the bottom left panel show that the endocytic vesicle membranes have been disrupted by the NIR irradiation. Higher magnification images with schematics show that some parts of the endocytic vesicles have disappeared, resulting in endolysosomal escape of the HGN to the cytosol. Adapted and reproduced with permission from AACR from Figure 2 of Lu et al. Cancer Research. 2010;70:3177–88 (10).