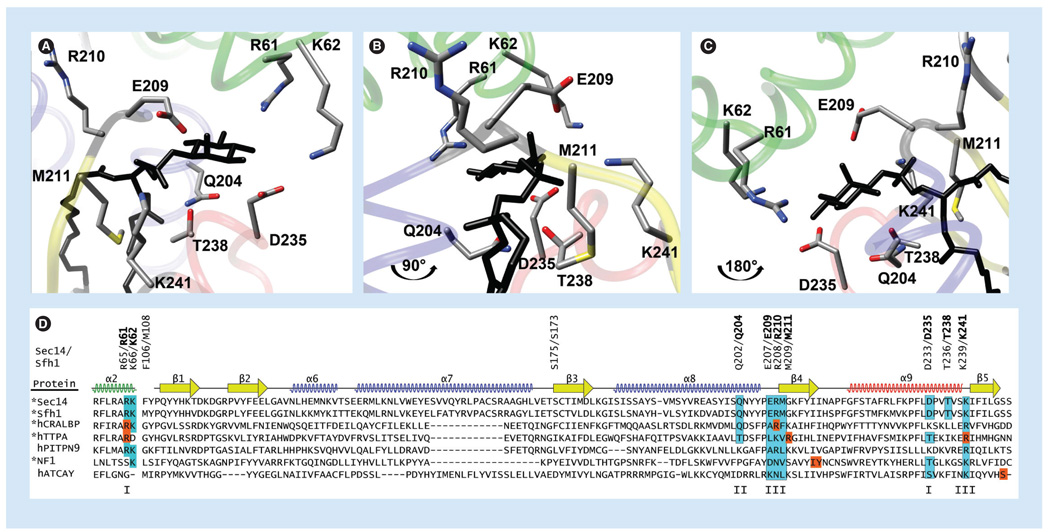
Figure 4. The phosphatidylinositol-binding barcode in Sec14-like proteins.
(A) Crystal structure of Sfh1 bound to phosphatidylinositol (PtdIns; black; 3B7N) highlighting residues within the PtdIns binding barcode. The tripod-motif is in green, the floor of the hydrophobic pocket is in yellow, and the α-helices are in blue with the exception of the helical gate, which is in red. (B) Orientation of the Sfh1 molecule is rotated by 90° counterclockwise parallel to the floor. (C) Orientation of the Sfh1 molecule is rotated by 180° counterclockwise parallel to the floor. (D) ClustalX2 alignments of selected Sec14-superfamily members (identified at right; proteins whose crystal structures have been solved are indicated with an ‘*’) were superimposed onto the Sfh1 crystal structure using secondary structural elements as a guide (diagrammed at top). Residues critical for PtdIns headgroup and backbone coordination are boxed and shaded in cyan – I, coordinate the Ins-headgroup; II, coordinate the glycerol backbone; III, coordinate the phosphate moiety through which the Ins headgroup is esterified to the glycerol backbone. Positions of missense substitution within the PtdIns-binding barcode of the corresponding Sec14-like protein that cause disease are highlighted by orange boxes.