Abstract
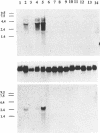
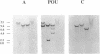
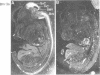
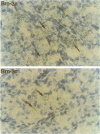
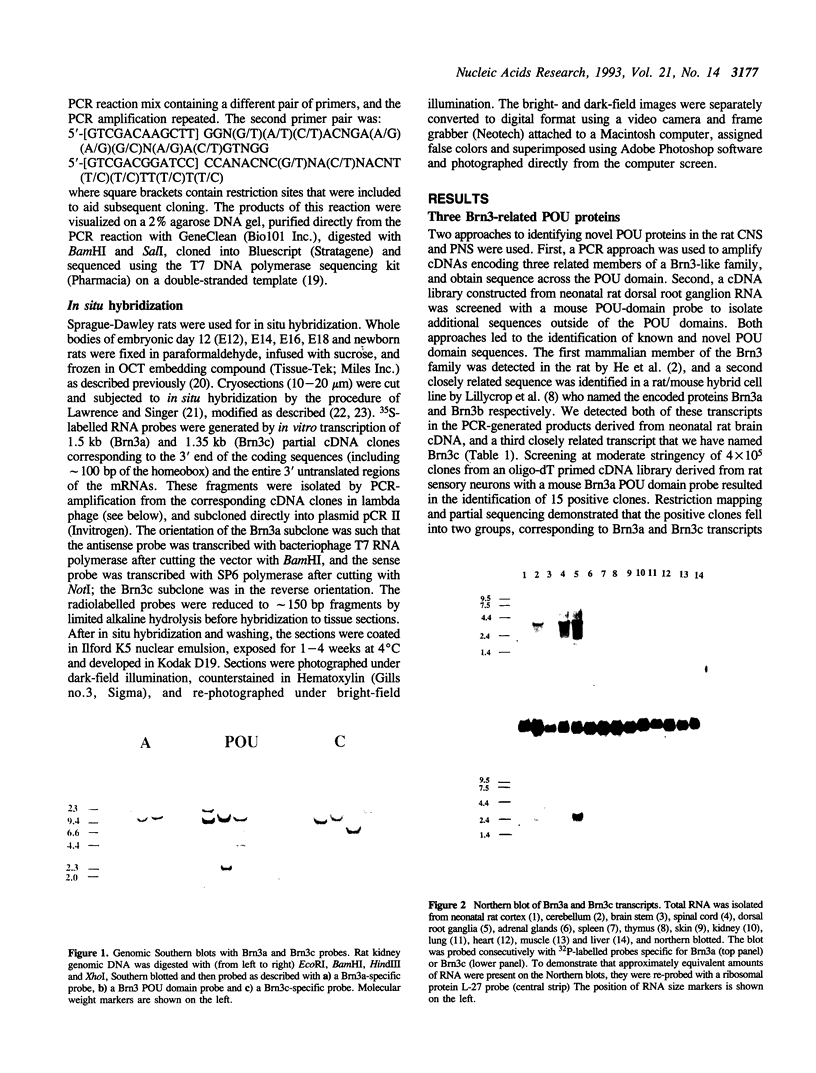
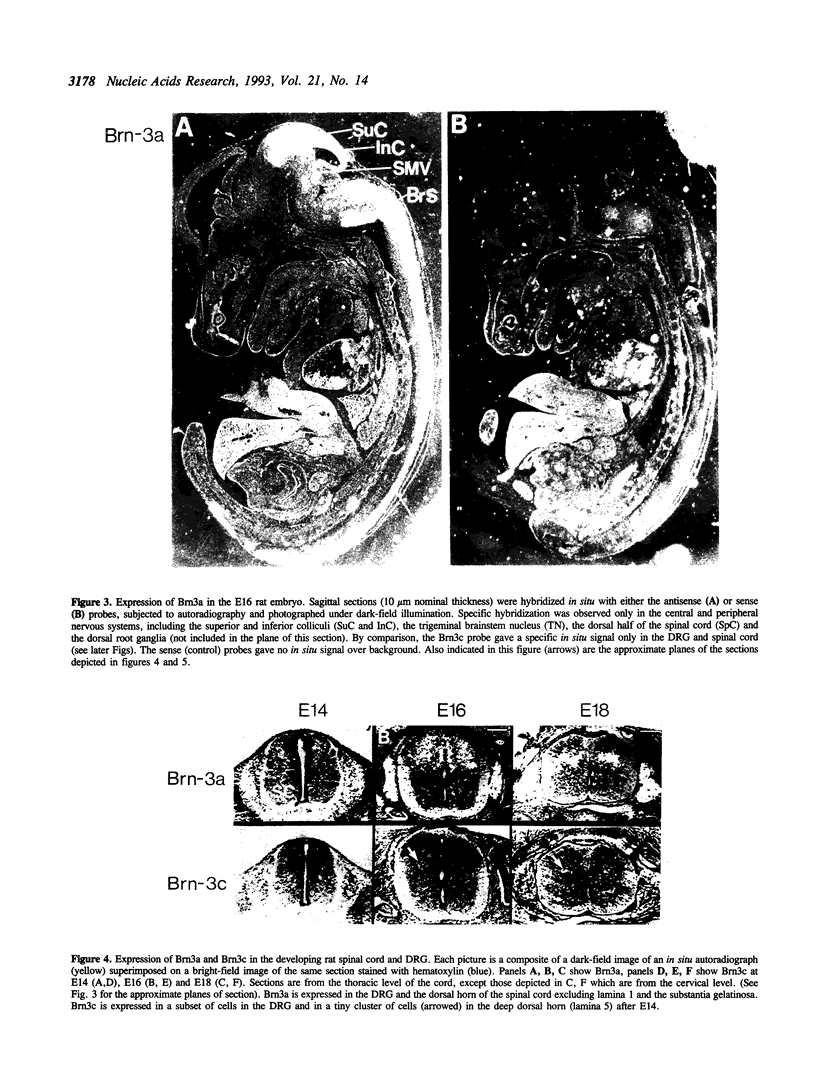
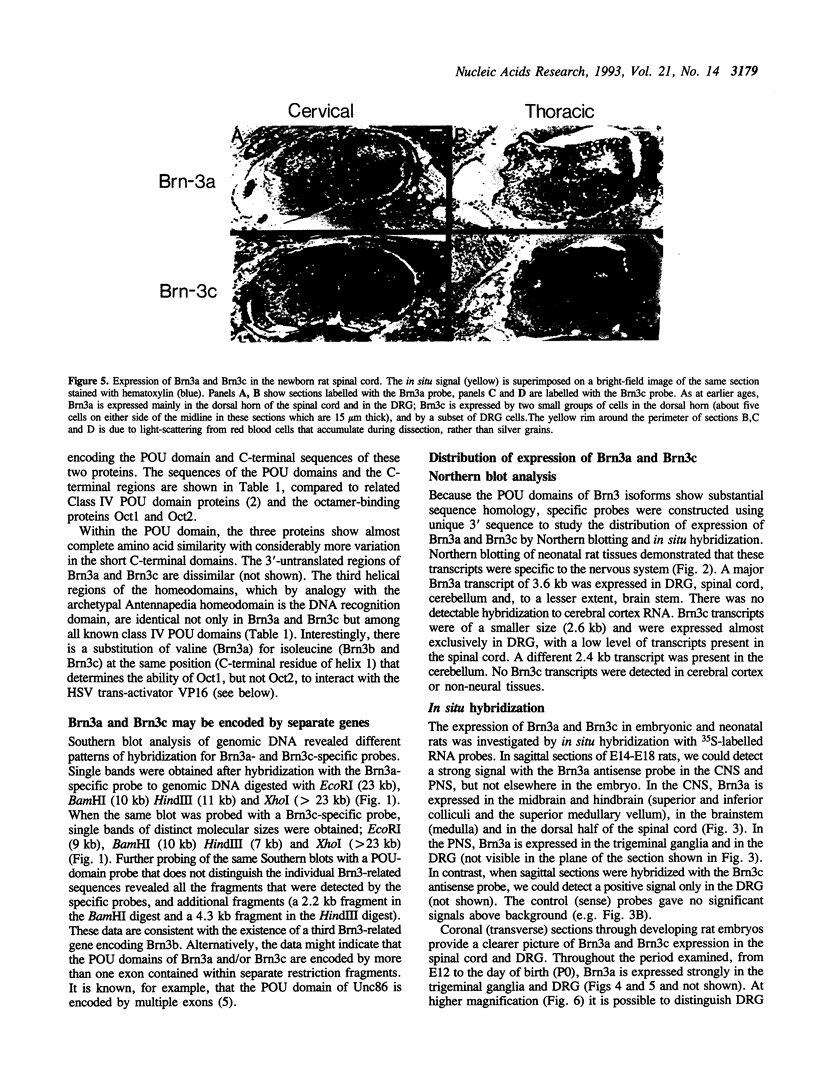
Brn3a and Brn3b are mammalian members of the POU class of transcription factors. They are closely related to each other and to Unc86, which determines the normal development of certain cells, including mechanoreceptive sensory neurons in Caenorhabditis elegans. We screened a rat dorsal root ganglion (DRG) cDNA library at moderate stringency with a Brn3a POU-domain probe and identified a novel transcript encoding a POU protein that we have named Brn3c. Brn3c closely resembles Brn3a and Brn3b in its POU-domain and thus helps define a family of Unc86-related mammalian POU factors. Both Brn3a and Brn3c are expressed only in the central and peripheral nervous systems. In the neonatal rat, northern blots revealed a 3.6 kb Brn3a transcript in DRG, spinal cord and hindbrain, and a 2.6 kb Brn3c transcript in DRG and spinal cord. In situ hybridization showed that most DRG neurons express Brn3a whereas only a small subset of neurons expresses Brn3c. In the spinal cord, Brn3a is expressed by many dorsal horn neurons. In contrast, Brn3c is expressed by a very small number of cells in laminae 4/5 of the dorsal horn. These data suggest that Brn3-related POU factors may be involved in the development or function of particular subclasses of sensory and spinal cord neurons.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collum R. G., Fisher P. E., Datta M., Mellis S., Thiele C., Huebner K., Croce C. M., Israel M. A., Theil T., Moroy T. A novel POU homeodomain gene specifically expressed in cells of the developing mammalian nervous system. Nucleic Acids Res. 1992 Sep 25;20(18):4919–4925. doi: 10.1093/nar/20.18.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney M., Ruvkun G., Horvitz H. R. The C. elegans cell lineage and differentiation gene unc-86 encodes a protein with a homeodomain and extended similarity to transcription factors. Cell. 1988 Dec 2;55(5):757–769. doi: 10.1016/0092-8674(88)90132-8. [DOI] [PubMed] [Google Scholar]
- Goldsborough A., Ashworth A., Willison K. Cloning and sequencing of POU-boxes expressed in mouse testis. Nucleic Acids Res. 1990 Mar 25;18(6):1634–1634. doi: 10.1093/nar/18.6.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Rosenfeld M. G. Mechanisms of complex transcriptional regulation: implications for brain development. Neuron. 1991 Aug;7(2):183–196. doi: 10.1016/0896-6273(91)90257-z. [DOI] [PubMed] [Google Scholar]
- Herr W., Sturm R. A., Clerc R. G., Corcoran L. M., Baltimore D., Sharp P. A., Ingraham H. A., Rosenfeld M. G., Finney M., Ruvkun G. The POU domain: a large conserved region in the mammalian pit-1, oct-1, oct-2, and Caenorhabditis elegans unc-86 gene products. Genes Dev. 1988 Dec;2(12A):1513–1516. doi: 10.1101/gad.2.12a.1513. [DOI] [PubMed] [Google Scholar]
- Lai J. S., Cleary M. A., Herr W. A single amino acid exchange transfers VP16-induced positive control from the Oct-1 to the Oct-2 homeo domain. Genes Dev. 1992 Nov;6(11):2058–2065. doi: 10.1101/gad.6.11.2058. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H. Quantitative analysis of in situ hybridization methods for the detection of actin gene expression. Nucleic Acids Res. 1985 Mar 11;13(5):1777–1799. doi: 10.1093/nar/13.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeau M. C., Alvarez-Bolado G., Braissant O., Wahli W., Catsicas S. Ribosomal protein L27 is identical in chick and rat. Nucleic Acids Res. 1991 Mar 25;19(6):1337–1337. doi: 10.1093/nar/19.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillycrop K. A., Budrahan V. S., Lakin N. D., Terrenghi G., Wood J. N., Polak J. M., Latchman D. S. A novel POU family transcription factor is closely related to Brn-3 but has a distinct expression pattern in neuronal cells. Nucleic Acids Res. 1992 Oct 11;20(19):5093–5096. doi: 10.1093/nar/20.19.5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz J. L., Kristie T. M., Sharp P. A. Recognition of the surface of a homeo domain protein. Genes Dev. 1992 Nov;6(11):2047–2057. doi: 10.1101/gad.6.11.2047. [DOI] [PubMed] [Google Scholar]
- Pringle N. P., Mudhar H. S., Collarini E. J., Richardson W. D. PDGF receptors in the rat CNS: during late neurogenesis, PDGF alpha-receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development. 1992 Jun;115(2):535–551. doi: 10.1242/dev.115.2.535. [DOI] [PubMed] [Google Scholar]
- Pringle N. P., Richardson W. D. A singularity of PDGF alpha-receptor expression in the dorsoventral axis of the neural tube may define the origin of the oligodendrocyte lineage. Development. 1993 Feb;117(2):525–533. doi: 10.1242/dev.117.2.525. [DOI] [PubMed] [Google Scholar]
- Pringle N., Collarini E. J., Mosley M. J., Heldin C. H., Westermark B., Richardson W. D. PDGF A chain homodimers drive proliferation of bipotential (O-2A) glial progenitor cells in the developing rat optic nerve. EMBO J. 1989 Apr;8(4):1049–1056. doi: 10.1002/j.1460-2075.1989.tb03472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R., Hatzopoulos A. K., Balling R., Suzuki N., Gruss P. A family of octamer-specific proteins present during mouse embryogenesis: evidence for germline-specific expression of an Oct factor. EMBO J. 1989 Sep;8(9):2543–2550. doi: 10.1002/j.1460-2075.1989.tb08392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. N., Bevan S. J., Coote P. R., Dunn P. M., Harmar A., Hogan P., Latchman D. S., Morrison C., Rougon G., Theveniau M. Novel cell lines display properties of nociceptive sensory neurons. Proc Biol Sci. 1990 Sep 22;241(1302):187–194. doi: 10.1098/rspb.1990.0084. [DOI] [PubMed] [Google Scholar]
- Wood J. N., Lillycrop K. A., Dent C. L., Ninkina N. N., Beech M. M., Willoughby J. J., Winter J., Latchman D. S. Regulation of expression of the neuronal POU protein Oct-2 by nerve growth factor. J Biol Chem. 1992 Sep 5;267(25):17787–17791. [PubMed] [Google Scholar]