Abstract
Traditional models of sexual selection posit that male courtship signals evolve as indicators of underlying male genetic quality. An alternative hypothesis is that sexual conflict over mating generates antagonistic coevolution between male courtship persistence and female resistance. In the scarabaeine dung beetle Onthophagus taurus, females are more likely to mate with males that have high courtship rates. Here, we examine the effects of exposing females to males with either high or low courtship rates on female lifetime productivity and offspring viability. Females exposed to males with high courtship rates mated more often and produced offspring with greater egg–adult viability. Female productivity and lifespan were unaffected by exposure to males with high courtship rates. The data are consistent with models of sexual selection based on indirect genetic benefits, and provide little evidence for sexual conflict in this system.
Keywords: female choice, genetic benefits, sexual conflict, courtship rate
1. Introduction
Traditional models of sexual selection predict that male courtship displays signal genetic quality, and females that choose to mate with males capable of intense courtship displays accrue fitness benefits for their offspring in the form of enhanced attractiveness of sons and/or general offspring viability (see reviews in [1,2]). An alternative view is that male courtship displays evolve via sexual conflict. Because exposure to courtship [3] and mating [4] can be independently costly for females, female fitness can be reduced by male courtship displays per se, and by their effects on female mating rate. Antagonistic coevolution between males and females should generate cycles of adaptation in which males evolve to exceed female mating thresholds and females evolve to become resistant to male courtship [5,6]. Traditional models of sexual selection and sexual conflict thus make contrasting predictions concerning the costs and benefits of exposure to males delivering high levels of courtship; under conventional sexual selection, females should benefit from increased offspring fitness, while under sexual conflict these females should suffer greater costs of courtship and mating, and reduced fitness.
Scarabaeine dung beetles in the genus Onthophagus exhibit condition-dependent courtship behaviour. Males drum the female's dorsum with their fore- and mid-legs, and females show a greater probability of mating with males capable of courting at a high rate [7]. In Onthophagus taurus, courtship rate exhibits considerable additive genetic variation, and is genetically correlated with general body condition [7]. Moreover, there are genetic correlations between condition and reproductive traits that contribute to a male's competitive fertilization success: testes size and sperm length [8,9]. The genetic architecture of both pre- and post-copulatory sexual traits thereby predicts that females will obtain indirect benefits for their offspring when mating with males delivering high courtship rates. However, female benefits might be outweighed by mating costs if these males displace females from their optimal mating rate [10]. Here, we experimentally manipulate the quality of a female's mates by pre-screening males, and examine the relative costs and benefits to females of exposure to males that differ in their courtship rates.
2. Material and methods
Beetles used in these experiments were bred in the laboratory following established protocols [11]. Sexes were separated on emergence to ensure they remained unmated, and were provided ad libitum access to fresh dung for 2 weeks prior to their use in experiments.
We screened 119 males for courtship rate. Males were placed into artificial tunnels, 1 × 2 × 5 cm with a plaster-of-Paris floor that had been moistened with fresh dung. A single unmated female was introduced into each male's tunnel and the pair observed for 60 min under red light, or until the pair mated. We checked each pair every 2 min and noted whether the male was courting. Courtship rate was calculated as the number of observations in which he was seen to be courting, divided by the total time the pair were observed (60 mins or less if the pair mated [7,12]). The courtship rate of each male was determined three times, with three different females. Courtship rate was significantly repeatable (F118,238 = 1.88, p < 0.0001; R = 0.227 ± 0.059; calculated following [13]). The average courtship rate for each male was calculated, and the males ranked from high to low. We selected males from the extremes of the courtship rate distribution (45 high and 39 low) to use in our experimental treatments.
A total of 86 females were assigned haphazardly to one of two treatments whereby females were exposed to males with either high or low courtship rates. Each day, for 6 days, females were introduced into artificial tunnels together with a male from the appropriate treatment, and the pair observed for 60 mins. We recorded courtship rates during these trials, and noted whether the female mated or not. Males were used more than once, but never with the same female, and were always rested for 24 h between trials. After the 6 day exposure to males, females were placed into individual breeding chambers (plastic piping, 9 cm in diameter, three quarters filled with moist sand, and topped with 250 ml fresh cow dung), and left for 7 days to produce broods. Breeding chambers were sieved and the broods collected. Loose sand was removed from broods, and they were weighed to an accuracy of 0.1 g. Broods were re-buried in moist sand within plastic containers, and incubated for 3 weeks until adult beetles emerged. After sieving, females were re-established in a new breeding chamber and left for a further 7 days. The process was thus repeated weekly until the female died, and the total number of broods was summed to give a measure of lifetime brood productivity. Egg–adult viability was scored by noting whether an adult beetle emerged from its brood.
3. Results
The courtship rates (CRT) of males to which females in the two treatments were exposed differed significantly (mean (×10−3) CRT experienced by females in the high CRT treatment, 3.9 ± 0.2 bouts per minute; low CRT treatment, 2.9 ± 0.2; F1,508 = 19.20, p < 0.0001). Females exposed to males with high courtship rates mated more rapidly (F1,84 = 10.76, p = 0.0015) and with more of the males (F1,84 = 10.22, p < 0.0001) than females exposed to males with low courtship rates (table 1). The number of mates accepted (mating frequency) was included as a covariate in subsequent analyses.
Table 1.
Mating behaviour, reproductive output and longevity of females exposed to males with high and low courtship rates (means ± 1 s.e., range in parenthesis).
| high CRT | low CRT | |
|---|---|---|
| mating speed (min) | 32.9 ± 1.9 (4–60) | 41.1 ± 1.9 (19.6–56.1) |
| mating frequency | 3.4 ± 0.2 (1–6) | 2.5 ± 0.2 (1–5) |
| number of broods | 12.0 ± 1.4 (0–38) | 13.0 ± 1.4 (0–42) |
| brood weight (g) | 4.2 ± 0.2 (1.9–6.3) | 4.2 ± 0.2 (2.9–7.3) |
| longevity (weeks) | 3.0 ± 0.2 (1–5) | 2.8 ± 0.2 (1–5) |
There were no significant effects of CRT treatment or female mating frequency on the lifetime number of broods that females produced (CRT treatment: F1,83 = 1.03, p = 0.314; mating frequency, F1,83 = 2.37, p = 0.127; table 1). The interaction between CRT treatment and mating frequency was not significant (F1,82 = 2.65, p = 0.108) and removed from the model. Likewise, there was no effect of CRT treatment or mating frequency on the mean weight of broods (CRT treatment: F1,83 = 0.14, p = 0.707; mating frequency, F1,83 = 0.29, p = 0.592; table 1). The interaction between CRT treatment and mating frequency was not significant (F1,82 = 3.44, p = 0.068). Nevertheless, the p-value associated with the interaction term was low, and we note that brood weight tended to increase with mating frequency when females were exposed to high quality males, but tended to decrease with mating frequency when females were exposed to low quality males.
We used a Cox-regression to examine variation in female lifespan. There was no significant effect of CRT treatment (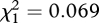 , p = 0.793) or mating frequency (
, p = 0.793) or mating frequency (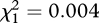 , p = 0.949), and no interaction effect (
, p = 0.949), and no interaction effect (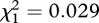 , p = 0.864) on female lifespan (table 1).
, p = 0.864) on female lifespan (table 1).
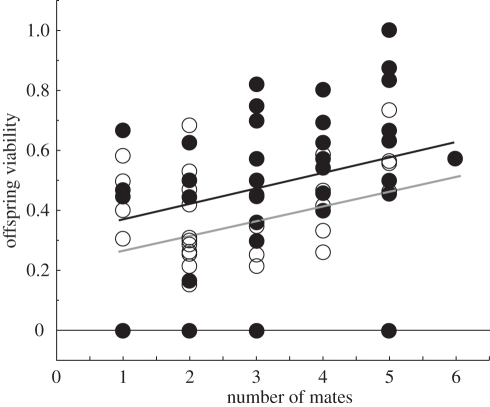
Egg–adult offspring viability was analysed using a generalized linear model, using number of offspring for each female that survived to adult emergence as the dependent variable, the number of broods as the binomial denominator, and a logit link function. The data were overdispersed, so we used F-tests, rather than χ2, to test statistical significance [14]. The interaction between CRT treatment and mating frequency was not significant (F1,74 = 1.24, p = 0.269), and was removed from the model. Offspring viability increased with female mating frequency (F1,75 = 5.82, p = 0.018), and females mated to high CRT males produced offspring of higher viability than females mated to low CRT males (F1,75 = 5.99, p = 0.017; figure 1).
Figure 1.
Effect of the number of males a female mated with and male quality on offspring viability (proportion of broods from which an adult beetle emerged). Females exposed to males with high courtship rates are depicted as solid symbols with a black least squares slope, and those exposed to males with low courtship rates are depicted as open symbols with a grey least squares slope.
4. Discussion
Female O. taurus show a mating preference for males with high courtship rates [7], and when exposed to such males, they mated sooner and had a higher mating frequency than females exposed to males with low courtship rates. We found significant positive effects of mating frequency on female fitness. The egg–adult viability of offspring increased with female mating frequency, and females mated to males delivering high courtship rates produced more viable offspring, over and above the effects of mating frequency. Neither mating frequency nor male courtship rate influenced a female's brood productivity or lifespan. Thus, mating and exposure to males with high courtship rates had no apparent costs. Mating with high quality males may generate fitness costs in subsequent generations, for example, if sons or daughters had reduced sexual fitness, as seems to be the case for seed beetles [15]. However, the current evidence for O. taurus suggests that conventional good-genes models of sexual selection are likely to play an important role in the evolution of polyandry and female choice.
There are two potential mechanisms for the effect of mating frequency on offspring viability. Females may acquire direct benefits associated with mating, such as paternal contributions within the seminal fluid that enhance offspring viability [16]. Alternatively, increased mating frequency may afford females a greater ability to avoid genetic incompatibilities. Bilde et al. [17] have found both additive and non-additive genetic effects on offspring fitness in seed beetles. Whatever the underlying mechanism of the mating frequency effect, the independent effect of male quality on offspring viability provides evidence for indirect genetic benefits associated with pre-copulatory female choice based on male courtship rate.
Pre- and post-copulatory sexual traits of male O. taurus both exhibit high levels of additive genetic variance and are genetically correlated with general body condition [7,8], thereby providing the appropriate genetic architecture for good-genes sexual selection. However, non-genetic maternal effects could potentially contribute to the patterns of variation in offspring viability that we report here. If females increase their investment in reproduction when mating with high quality males, then differential maternal allocation could account for the greater egg–adult viability [18]. We noted that females mated with high quality males tended to increase brood weight, though not significantly, with increased mating frequency, and small increments in brood provisions could have strong effects on the growth and development of offspring. Previous work has shown how differential maternal provisioning can inflate additive genetic effects on offspring size in this species [19]. The magnitude of additive genetic variance (CVA) in egg–adult viability has recently been estimated to be in the region of 15 per cent (F. García-González & L. W. Simmons 2010, unpublished data). However, studies that control genetic and maternal contributions to offspring performance are required to ascribe benefits of polyandry unequivocally to indirect genetic effects [20]. In such an experiment, Simmons [9] allowed females to mate with both high and low condition males, so that females did not differ in their experience of mating partners. However, for each female, one of her mates had been selected at random to be exposed to ionizing radiation that resulted in his offspring failing to develop. The results demonstrated that, when potential differences in maternal effects on offspring viability were controlled, males of high condition sired offspring of high condition, which had a greater probability of surviving to reproductive maturity. The data for O. taurus thereby provide compelling support for good-genes processes operating at both pre- and post-copulatory episodes of sexual selection [9].
Acknowledgements
This research was supported by the Australian Research Council. We thank Paco García-González for discussion, and comments on the manuscript.
References
- 1.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Kokko H., Jennions M. D., Brooks R. 2006. Unifying and testing models of sexual selection. Annu. Rev. Ecol. Evol. Syst. 37, 43–66 10.1146/annurev.ecolsys.37.091305.110259 (doi:10.1146/annurev.ecolsys.37.091305.110259) [DOI] [Google Scholar]
- 3.Partridge L., Fowler K. 1990. Non-mating costs of exposure to males in female Drosophila melanogaster. J. Insect Physiol. 36, 419–425 10.1016/0022-1910(90)90059-O (doi:10.1016/0022-1910(90)90059-O) [DOI] [Google Scholar]
- 4.Chapman T., Liddle L. F., Kalb J. M., Wolfner M. F., Partridge L. 1995. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 373, 241–244 10.1038/373241a0 (doi:10.1038/373241a0) [DOI] [PubMed] [Google Scholar]
- 5.Parker G. A. 1979. Sexual selection and sexual conflict. In Sexual selection and reproductive competition in insects (eds Blum M. S., Blum N. A.), pp. 123–166 London, UK: Academic Press [Google Scholar]
- 6.Holland B., Rice W. R. 1998. Chase-away sexual selection: antagonistic seduction versus resistance. Evolution 52, 1–7 10.2307/2410914 (doi:10.2307/2410914) [DOI] [PubMed] [Google Scholar]
- 7.Kotiaho J. S., Simmons L. W., Tomkins J. L. 2001. Towards a resolution of the lek paradox. Nature 410, 684–686 10.1038/35070557 (doi:10.1038/35070557) [DOI] [PubMed] [Google Scholar]
- 8.Simmons L. W., Kotiaho J. S. 2002. Evolution of ejaculates: patterns of phenotypic and genotypic variation and condition dependence in sperm competition traits. Evolution 56, 1622–1631 10.1554/0014-3820(2002)056[1622:EOEPOP]2.0.CO;2 (doi:10.1554/0014-3820(2002)056[1622:EOEPOP]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 9.Simmons L. W. 2011. Sexual selection after mating: the evolutionary consequences of sperm competition and cryptic female choice in onthophagines. In Ecology and evolution of dung beetles (eds Simmons L. W., Ridsdill-Smith J.). Oxford, UK: Wiley-Blackwell [Google Scholar]
- 10.Cameron E., Day T., Rowe L. 2003. Sexual conflict and indirect benefits. J. Evol. Biol. 16, 1055–1060 10.1046/j.1420-9101.2003.00584.x (doi:10.1046/j.1420-9101.2003.00584.x) [DOI] [PubMed] [Google Scholar]
- 11.Simmons L. W., García-González F. 2008. Evolutionary reduction in testes size and competitive fertilization success in response to the experimental removal of sexual selection in dung beetles. Evolution 62, 2580–2591 10.1111/j.1558-5646.2008.00479.x (doi:10.1111/j.1558-5646.2008.00479.x) [DOI] [PubMed] [Google Scholar]
- 12.Kotiaho J. S. 2002. Sexual selection and condition dependence of courtship display in three species of horned dung beetle. Behav. Ecol. 13, 791–799 10.1093/beheco/13.6.791 (doi:10.1093/beheco/13.6.791) [DOI] [Google Scholar]
- 13.Becker W. A. 1984. Manual of quantitative genetics. Washington, DC: Pullman [Google Scholar]
- 14.Crawley M. J. 1993. GLIM for ecologists. Cambridge, MA: Blackwell [Google Scholar]
- 15.Bilde T., Foged A., Schilling N., Arnqvist G. 2009. Postmating sexual selection favors males that sire offspring with low fitness. Science 324, 1705–1706 10.1126/science.1171675 (doi:10.1126/science.1171675) [DOI] [PubMed] [Google Scholar]
- 16.García-González F., Simmons L. W. 2007. Paternal indirect genetic effects on offspring viability and the benefits of polyandry. Curr. Biol. 17, 32–36 10.1016/j.cub.2006.10.054 (doi:10.1016/j.cub.2006.10.054) [DOI] [PubMed] [Google Scholar]
- 17.Bilde T., Friberg U., Maklakov A. A., Fry J. D., Arnqvist G. 2008. The genetic architecture of fitness in a seed beetle: assessing the potential for indirect genetic benefits of female choice. BMC Evol. Biol. 8, 295. 10.1186/1471-2148-8-295 (doi:10.1186/1471-2148-8-295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheldon B. C. 2000. Differential allocation: tests, mechanisms and implications. Trends. Ecol. Evol. 15, 397–402 10.1016/S0169-5347(00)01953-4 (doi:10.1016/S0169-5347(00)01953-4) [DOI] [PubMed] [Google Scholar]
- 19.Kotiaho J. S., Simmons L. W., Hunt J., Tomkins J. L. 2003. Males influence maternal effects that promote sexual selection: a quantitative genetic experiment with dung beetles Onthophagus taurus. Am. Nat. 161, 852–859 10.1086/375173 (doi:10.1086/375173) [DOI] [PubMed] [Google Scholar]
- 20.Kozielska M., Krzeminska A., Radwan J. 2003. Good genes and the maternal effects of polyandry on offspring reproductive success in the bulb mite. Proc. R. Soc. Lond. B 271, 165–170 10.1098/rspb.2003.2585 (doi:10.1098/rspb.2003.2585) [DOI] [PMC free article] [PubMed] [Google Scholar]