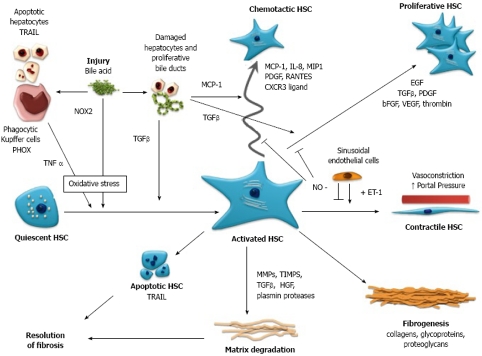
Figure 1.
Schematic representation of the activation, function and interaction of Hepatic Stellate Cells (HSC) with other cells of the liver in cholestatic liver injury. Bile acid mediated injury is proposed to impact on HSC directly and indirectly via oxidative stress mediated pathways, resulting in the transformation of quiescent HSC to an activated phenotype, i.e. myofibroblast. Activated HSC are proliferative and fibrogenic and are responsible for increased production and deposition of fibrillar collagens and extracellular matrix, leading to fibrosis and cirrhosis. In response to hepatocyte and cholangiocyte-derived chemokines, motile HSC are recruited to the site of injury along the growing margin of scar tissue, with HSC and portal myofibroblasts also demonstrated surrounding bile ducts. HSC assume a vasoconstrictive phenotype resulting in increased portal pressure. Hepatic fibrosis is ultimately resolvable with disease treatment or cessation of injury, as HSC produce fibrinolytic enzymes and are themselves subject to apoptosis as part of the process of fibrosis resolution. TRAIL: tumor necrosis factor-related apoptosis-inducing ligand; PHOX: phagocytic NADPH oxidase; NOX2: non-phagocytic NADPH oxidase; TNFα: tumor necrosis factor α; TGFβ: transforming growth factor β; MCP-1: monocyte chemotaxis protein-1; IL-8: interleukin-8; MIP1: macrophage inflammatory protein 1; PDGF: platelet derived growth factor; RANTES: regulated upon activation, normal T cell expressed and secreted; CXCR3 ligand: chemokine (C-X-C) receptor 3 ligand; EGF: epidermal growth factor; bFGF: basic fibroblast growth factor; VEGF: vascular endothelial growth factor; NO: nitric oxide; ET-1: endothelin 1; MMP-1: matrix metalloproteinase-1; TIMPS: tissue inhibitors of metalloproteinase; HGF: hepatocyte growth factor.