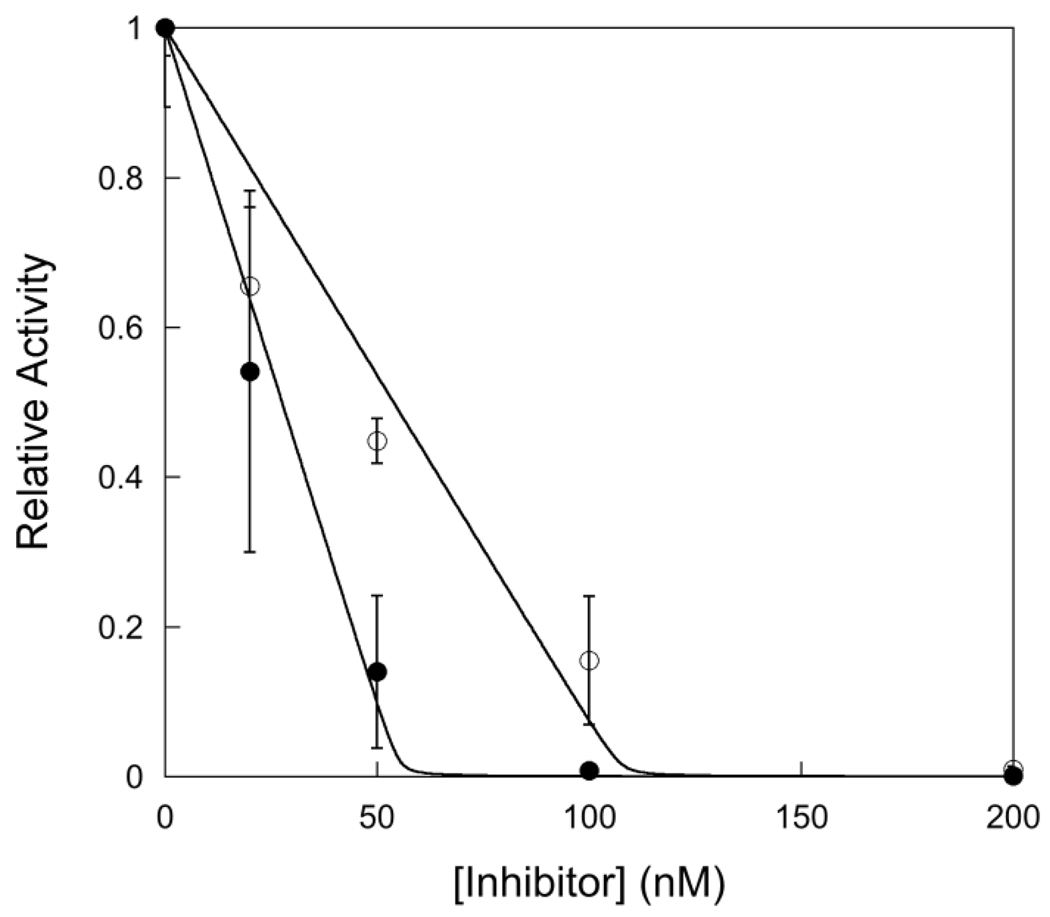
Figure 1.
Titration with a tight-binding pyrrolidine inhibitor to determine the concentration of active AlkA. Experiments were performed using 1 µM 19mer I•T substrate (19u) with varying concentrations (from 0 to 400 nM) of 25mer pyrrolidine inhibitor (Y•T). The concentration of AlkA was 100 nM (●) and 200 nM (○). The fraction of active AlkA was determined by measuring the initial rate of product formation and plotting the relative rate (Vobs/Vmax) versus the concentration of inhibitor. The average and standard deviation of two to four replicates are shown. This titration gives an average value of 0.57 ± 0.03 for the fraction of active AlkA, assuming a single monomer binds to each DNA (11).