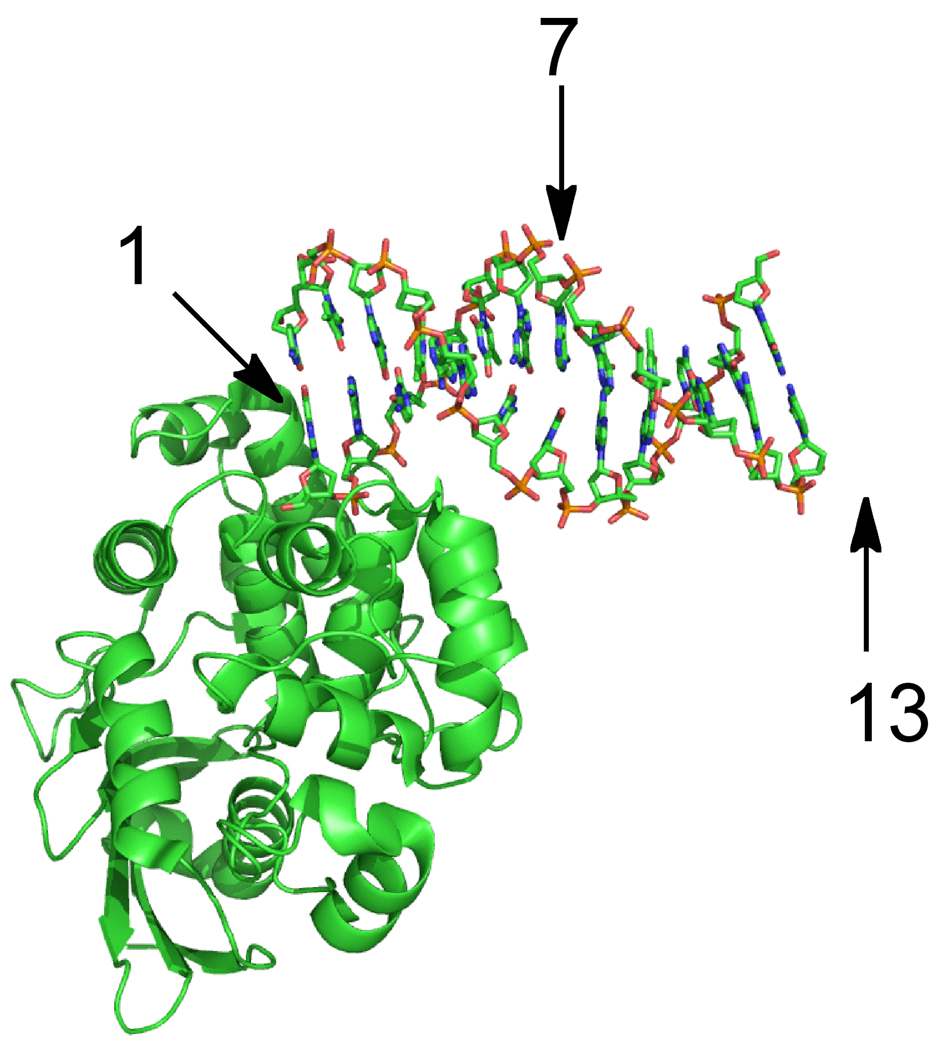
Figure 6.
Structure of AlkA bound to the end of a duplex. The figure was rendered with Pymol and the coordinates are from the Protein database (3CWT; (12)). This crystal form contains two oligonucleotides and four AlkA monomers per asymmetric unit, but only one AlkA-DNA interaction is shown. AlkA interacts predominantly with the strand that donates the 5’ end of the DNA. Arrows indicate the nucleotide position relative to the 5’end. Position 7 corresponds to the position of the lesion in the 19u substrate, and this lies on the opposite face of the DNA from a protein bound to the 5’ end. Position 13 (not present in the crystallized 12mer oligonucleotide) is on the same face as the end-bound AlkA molecule, but is separated by approximately one turn of the helix. This suggests that more than one AlkA molecule would need to bind to the 25mer symmetric substrate before the lesion site (position 13) would experience interference.