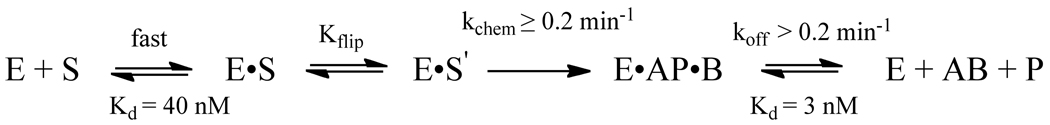
Figure 8.
Minimal kinetic mechanism for the recognition and excision of hypoxanthine by AlkA. The binding and flipping steps are assumed to be in rapid equilibrium, as has been seen for other glycosylases (32). The absence of a burst indicates that the release of Hx (B) and abasic DNA (AP) products are not rate limiting. However, AlkA shows potent inhibition by abasic sites. The rate of N-glycosidic bond cleavage must be at least as fast as the observed rate constant for the single turnover reaction, however it could be significantly faster if the flipping equilibrium is unfavorable (37).