Figure 1.
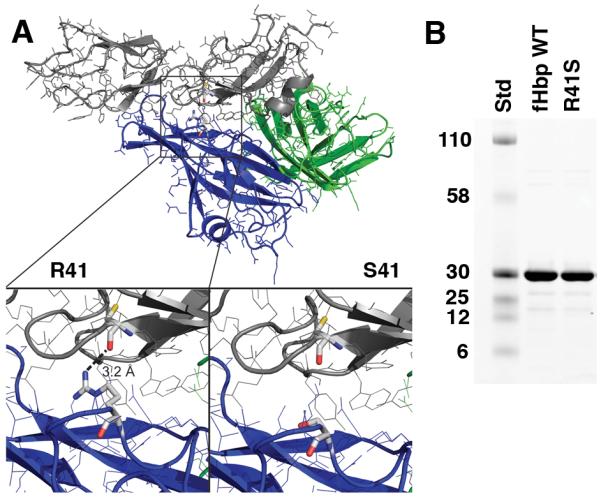
A, Structural model of fHbp bound to a fragment of fH based on published atomic coordinates (43). The blue and green ribbons represent the respective N- and C-terminal domains of the fHbp molecule. The gray ribbon represents the sixth and seventh short consensus repeat domains of human fH previously shown to mediate the interaction of human fH and fHbp (43). The arginine residue at position 41 formed a charged H-bond with fH (left lower inset), which was predicted to be eliminated when arginine was replaced by serine (right lower inset). B, SDS-PAGE of fHbp. Wild-type (WT) fHbp and R41S mutant fHbp (5 μg/lane) were separated on 4-12% polyacyrlamide gradient gel (NuPAGE, Invitrogen, Carlsbad, CA) and stained with Coomassie blue. The molecular weights (in kDa) of the protein standards (Std) are labeled on the left.
