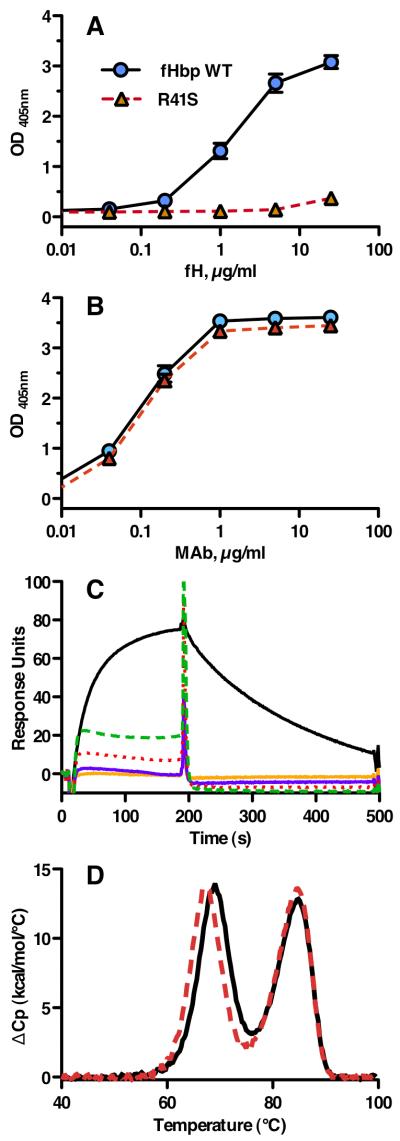
Figure 2.
Characterization of fHbp vaccine antigens. A, By ELISA, the fHbp WT (blue circles) bound fH, whereas the R41S substitution (red triangles, dashed line) eliminated binding of soluble human fH to solid-phase mutant fHbp. B, Binding of anti-fHbp MAb JAR 4 to solid-phase wild-type or R41S mutant fHbp indicated that similar amounts of the two proteins were adsorbed to the wells of the microtiter plate and that a conformational epitope in the N-terminal domain was retained. Symbols are the same as used in panel A. The data in panels A and B represent the mean and SE of three to six independent measurements. For data points without apparent error bars, the variability was too small to be evident. C, Binding of soluble fHbp to immobilized human fH as measured by surface plasmon resonance. Human fH (4000 response units) was coupled to the biosensor chip; the WT fHbp (0.5 μM, solid black line) showed +77.4 response units while 0.5 (solid orange line) or 1.0 μM (solid purple line) of the mutant R41S fHbp showed no binding (−1.0 and −0.8, respectively). Non-specific binding of the mutant fHbp is evident at 2.5 (red dotted line) or 5.0 (green dashed line) μM. D, Thermal stability of WT (solid black line) and R41S (dashed red line) proteins measured by differential scanning calorimetry. Protein solutions (0.5 mg/ml) were in PBS and the scan rate was 60 °C/h. Reference buffer data were subtracted as a baseline and the data were normalized based on the calculated molecular weight of the recombinant fHbp (27.7 kDa). The lower and higher temperature transitions correspond to the unfolding of the N- and C-terminal domains, respectively (46).