Abstract
Objectives
A community level randomised controlled trial of a Community Popular Opinion Leader (C-POL) intervention to reduce bacterial and viral sexually transmitted infections (STIs) and unprotected extramarital sex was carried out over 2 years in five countries. The main study results did not find significant intervention effects. This paper presents a sub-analysis examining the differential intervention impacts among high-risk and low-risk participants in the China site.
Methods
From 2002 – 2006, 3912 migrant market vendors aged 18 and 49 years were recruited at an urban site in China. Markets were randomly assigned to the C-POL intervention (N=20 markets; n=1979) or standard-care control condition (N=20; n=1933). Both study condition venues received HIV/STI education, free condoms, STI testing and treatment, and training for pharmacists in antibiotic treatments. In intervention markets, C-POLs were identified and trained to diffuse messages regarding safer sex, STI treatment and partner discussions of sex. The primary biological outcome was incidence of new STIs (chlamydia, gonorrhoea, syphilis, trichomonas, herpes or HIV). The primary sexual behaviour risk outcome was any unprotected extramarital sex in the prior 3 months.
Results
In unadjusted analyses, women had significantly lower rates of STI infection at 24 months in the C-POL intervention (5.7%) compared to controls (8.3%; p=0.043). In mixed-effects regression models, intervention participants with STIs at previous assessments were about half as likely to have STIs at 24 months (OR 0.47, 95% CI 0.25 to 0.90) compared to controls.
Conclusions
The C-POL intervention lowers HIV risk among those at highest risk (ie, with a STI or engaging in high-risk sexual activities) rather than the general population.
INTRODUCTION
The Community Popular Opinion Leader (C-POL) multisite intervention trial for HIV/sexually transmitted infection (STI) prevention was a Phase II, five-country collaboration conducted by local government health officials and researchers in China, India, Peru, Russia and Zimbabwe.1 The intervention aimed to modify community level social norms for safer sex behaviours using peer C-POLs as change agents to endorse and diffuse safe behaviour norms. The C-POL intervention targeted at-risk communities in each country and recruited large community based samples that included thousands of individual participants with a range of risk levels and STI histories. The recently published main study results that examined both aggregated data across the five countries and within country data showed no significant intervention effects for STI and behavioural outcome measures.2
The C-POL intervention’s positive outcomes in previous successful trials3,4 and the recent negative findings2 may be accounted for by differential impacts among the highest risk community members (ie, those with a STI or high levels of behavioural risk) compared to the much larger proportions of less risky participants in the samples. In addition, to ensure that only the impact of the C-POL intervention was evaluated and to meet ethical standards for conduct of research, both the control and intervention conditions received a substantial intervention comprised of STI diagnosis and antibiotic treatment services, access to condoms, STI/HIV educational materials and presentations, and ongoing monitoring through study assessments. This article presents a sub-analysis from the China site by the China site’s investigator team examining whether the C-POL intervention was efficacious in reducing STIs and behavioural risks among the highest risk participants: those with a STI or reporting recent unprotected extramarital sex compared to participants without non-spousal partners or a STI.
Hypotheses
We hypothesise that persons with STIs in the C-POL intervention will have significantly fewer STIs at the next follow-up compared to persons with STI in the control markets.
We hypothesise that persons reporting unprotected extramarital sex in the C-POL intervention previously will have significantly lower probability of unprotected extramarital sex at follow-ups compared to those in the control markets.
METHODS
Study background
The National Institute of Mental Health Collaborative HIV/STD Prevention Trial was conducted from 2002–2006 in China, India, Peru, Russia and Zimbabwe.1 The C-POL intervention is a community-level intervention that seeks to reduce STI and HIV risk by changing social norms at the community level based on Roger’s theory of the diffusion of innovations.5 The communities were selected to have sufficient baseline STI or rates of unprotected extramarital sex; population and network stability; organisational capacities (ie, political will of local health departments and stakeholders); sufficient numbers of sites for conducting a randomised trial; and sufficient distance between venues within country sites to minimise contamination.6 In China, these factors were explicitly considered when selecting migrant market vendors over other potential study populations, including construction workers, truck drivers, injection drug users, sex workers, men who have sex with men or factory workers.6
Participant recruitment and randomisation
Migrant food market vendors in a large Eastern coastal city in China were selected through a two-stage enumeration and random selection process. Markets in the site typically have 80–200 stalls and 150–300 owners and/or employees. Market venues were selected based on size and sufficient distances to prevent contamination across the intervention and control markets (ie, >2 km or a geographic barrier such as a river or highway). In total, 40 markets were selected from 95 possible markets.6 Box 1 describes the pre-trial activities to establish venue selection and study procedures.
Box 1 Pre-trial activities to establish venue selection and study procedures. C-POL.
Identify potential venues with observations and brief surveys (n=300 participants each): construction sites, factories, karaoke bars, rural villages, markets.
Select city and market venues; check for contamination possibilities across venues.
Map karaoke bars, sex work and beauty parlour establishments surrounding each market.
Survey and train pharmacists on current treatment strategies for STIs in city site.
Ethnographic studies on selection processes for community popular opinion leader (C-POLs); adapt HIV educational materials; risk situations; healthy norms regarding sexuality; segments of social groups.
Pilot C-POL training and reunion sessions.
Establish base rates of STI in venues with large epidemiological study in 40 markets; STI testing and treatment.
Match venues based on STI rates.
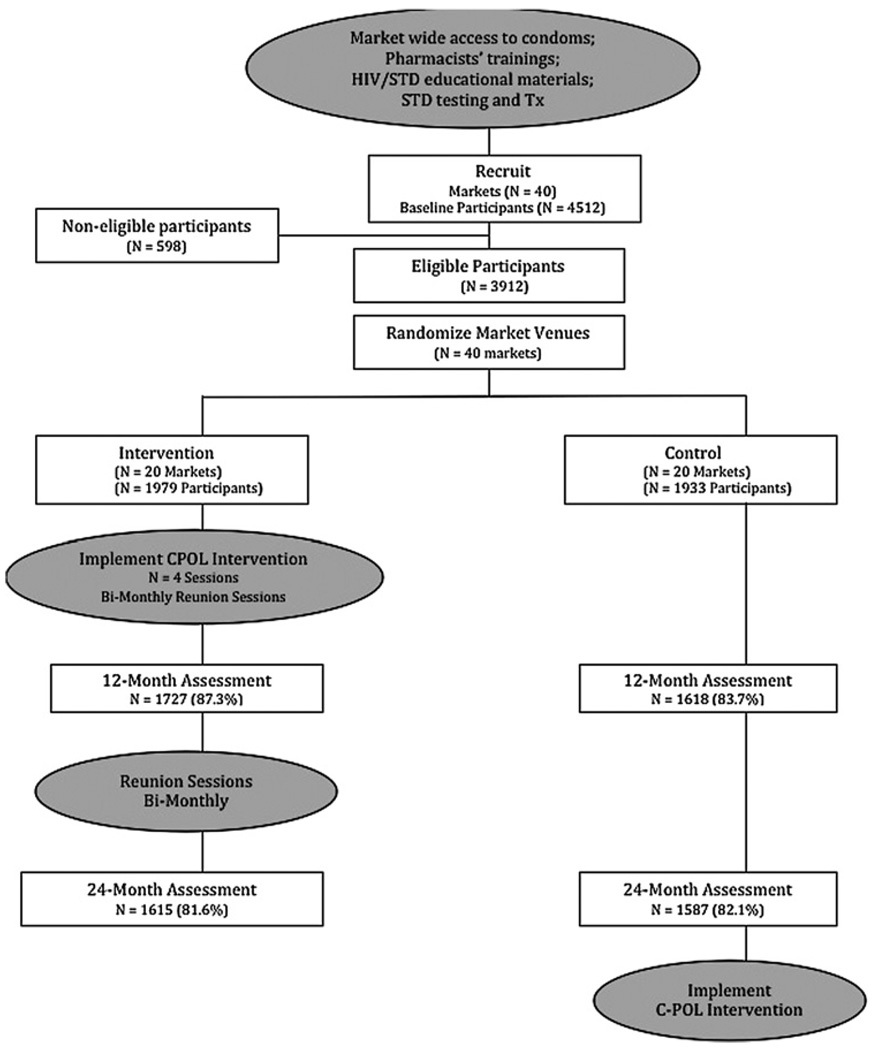
Randomisation proceeded in two phases. First, markets were paired based on a preliminary study of STI and behavioural risk conducted prior to intervention implementation.7 Sites were then randomly assigned to either the C-POL intervention or control condition by a Data Coordinating Center at the Research Triangle Institute with no investigator involvement. Second, a census of all stalls and employees was documented in the 40 markets. Potential participants were aged 18–49 years old and were briefly screened for eligibility based on whether they had engaged in sexual behaviour unprotected by condoms in the past 3 months. Randomisation schedules from the Data Coordinating Center identified the randomly selected stalls and a single eligible employee within each stall. Figure 1 shows the movement of participants through the trial.
Figure 1.
Participant flow through the trial at each major point.
Study procedures
A detailed description of the intervention design, methodology, protocol and procedures are presented elsewhere.1,8,9 Briefly, all participants provided written voluntary informed consent and completed baseline, 12-month and 24-month assessments that included a physical exam; STI/HIV testing from blood, urine and vaginal swabs; and syndromic STI diagnosis. The refusal rate for the initial recruitment was less than 8%. Interviewers collected self-report assessment data. Physicians conducted STI diagnosis and treatment at each assessment at a site separate from the markets. The interviewers and physicians did not know who was in the intervention or control conditions until after the 24-month assessment. Of 4512 eligible participants contacted and assessed at baseline, a total of 3914 (86.7%) was retained. About 20% of the population was selected to be C-POLs who were eligible to be randomly selected for study assessments at the intervention markets.
C-POL intervention procedures
C-POLs were identified through brief interviews conducted in intervention markets to identify social networks and to solicit nominations for C-POLs by market employees, managers, key informants and self-nominations. In addition, intervention trainers made repeated observations of the markets to identify popular employees. Based on Diffusion of Innovations theory,5 approximately 20% of the market vendors regularly present in each site were selected as C-POLs and invited to attend a series of four small-group training sessions. The sessions taught skills in delivering and diffusing theory based HIV/STI prevention messages to friends and acquaintances during everyday conversations. Each C-POL practiced diffusing prevention messages daily to peers and reported the frequency of conversations weekly. After the training sessions were completed, the C-POLs attended bimonthly reunion sessions to support sustained diffusion of prevention messages and to report on diffusion of messages to peers.
Study outcomes
The primary biological outcome was incidence of any new STI, including chlamydia, gonorrhoea, syphilis, trichomonas (females only), herpes (herpes simplex virus (HSV) 2) or HIV. All participants with a bacterial STI at baseline were treated. All STI diagnoses were based on laboratory confirmed testing (see online Appendix). Two composite binary variables were constructed: one indicating new infection with at least one of these six STIs and a second indicating a new bacterial STI infection (ie, excluding HSV and HIV). The composite variables were set to missing if more than one-third of a participant’s tests were either indeterminate or not done and there was no new positive test. The primary sexual behaviour risk outcome was defined as any unprotected sex with non-spousal partners in the past 3 months.
Power calculations
The five country C-POL trial was designed so that each country would have at least 80% power with a type I error rate of 5% (2-sided) to detect a 33% lower STI incidence in the intervention versus control, a participation rate of 95% and a follow-up rate of 84%. For the China site, we estimated 40 markets (20 matched pairs of markets) and 124 participants per market for statistical power. A detailed description of the power calculations for the multisite trial was published previously.1
Statistical analysis
We used the Transition Model10—one of the three commonly used longitudinal models in which the outcome variables are modelled as a function of immediate past outcome measures and explanatory variables—to test the hypothesis that intervention effects on STI incidence would be significantly greater for participants who had a STI at baseline or an incident STI during the study. We conducted the same analyses examining behavioural risk reports at prior assessments instead of STI. We used a generalised estimating equation model to test the hypothesis that participants with a STI at baseline would report significantly greater reductions in unprotected extramarital sex in the C-POL intervention compared to control.
Baseline differences between intervention and control samples were tested using χ2 and t tests (or Wilcoxon rank tests) for categorical and continuous variables, respectively. ORs and 95% CIs for longitudinal models are presented. All analyses were performed using SAS version 9.2.
RESULTS
Demographic and baseline characteristics are summarised in table 1. The majority of participants were women (55%) aged 31–40 years old (44%) with a mean age of 36 years. Men were 1 year older in the intervention compared to control condition (35.3 vs 34.2). Almost 88% had less than a high school education and 9% had no education. Almost all women were married (97%), but fewer men were married (87%). About 87% had a regular income. No differences between the two study groups were found in education, marital status or regularly earning money.
Table 1.
Demographic characteristics of participants by intervention and control condition
| Variable | Intervention N = 1979 |
Control N = 1933 |
Total N = 3912 |
|---|---|---|---|
| Female | 1073 (54.2) | 1064 (55.0) | 2137 (54.6) |
| Age | |||
| ≤24 | 161 (8.1) | 175 (9.1) | 336 (8.6) |
| 25–30 | 371 (18.8) | 376 (19.5) | 747 (19.1) |
| 31–40 | 839 (42.4) | 864 (44.7) | 1703 (43.5) |
| >40 | 608 (30.7) | 518 (26.8) | 1126 (28.8) |
| Mean* (SD) | 35.9 (7.9) | 35.2 (7.7) | 35.6 (7.8) |
| Male*: mean (SD) | 35.3 (8.3) | 34.2 (8.1) | 34.8 (8.2) |
| Female: mean (SD) | 36.4 (7.5) | 36.1 (7.3) | 36.2 (7.4) |
| Education | |||
| None | 176 (8.9) | 181 (9.4) | 357 (9.1) |
| Primary | 760 (38.4) | 784 (40.6) | 1544 (39.5) |
| Junior High | 776 (39.2) | 743 (38.4) | 1519 (38.8) |
| Senior High | 256 (12.9) | 215 (11.1) | 471 (12.0) |
| College or higher | 11 (0.6) | 10 (0.5) | 21 (0.5) |
| Married | 1844 (93.2) | 1784 (92.3) | 3628 (92.7) |
| Men | 792 (87.4) | 755 (86.9) | 1547 (87.2) |
| Women | 1052 (98.0) | 1029 (96.7) | 2081 (97.4) |
| Regularly earn money | 1715 (86.7) | 1682 (87.0) | 3397 (86.8) |
| Men | 808 (89.2) | 771 (88.7) | 1579 (89.0) |
| Women | 907 (84.5) | 911 (85.6) | 1818 (85.1) |
p<0.05.
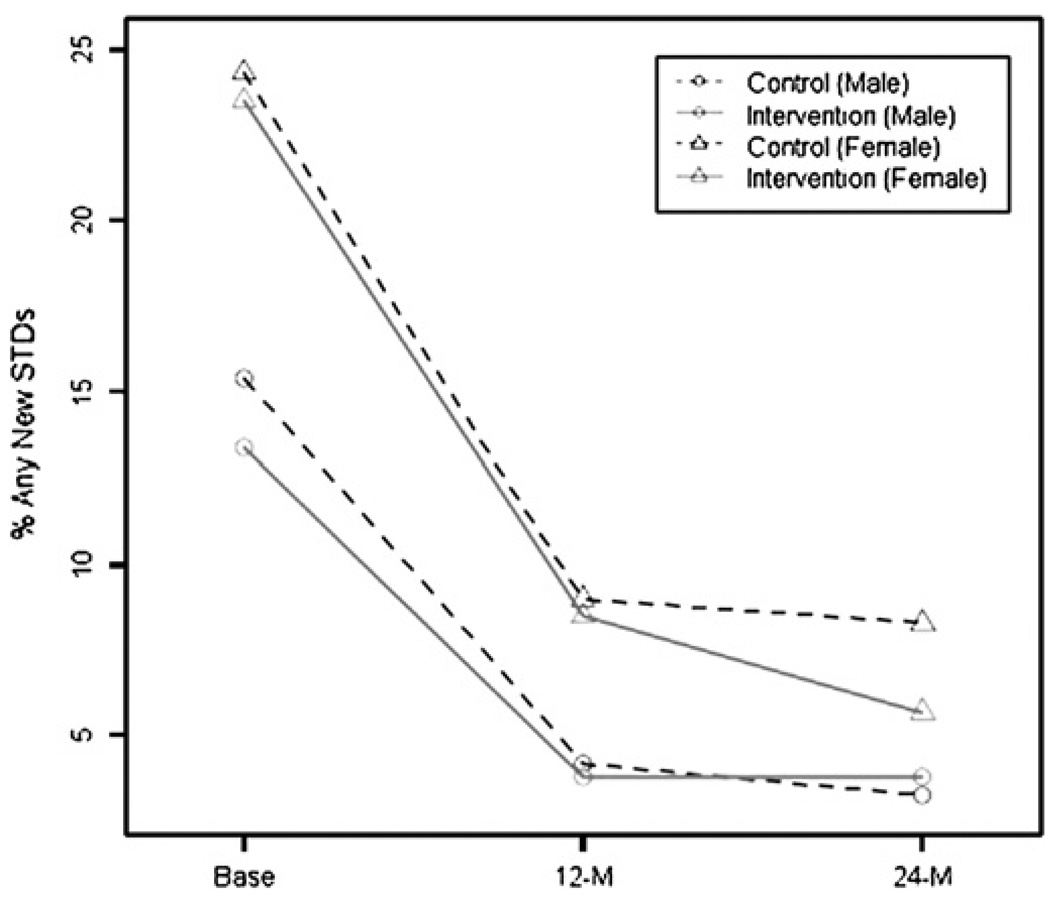
Table 2 shows a summary of behavioural and biological outcomes over time by gender and intervention condition. At baseline, only 6.5% of participants reported unprotected extramarital sex in the prior 3 months but about 20% had a STI. The disparity in baseline behavioural risk reports and STI infection rates was greatest among women: about 24% had an STI but only about 2.4% reported unprotected extramarital sex. In stark contrast, 14.4% of men had STIs of whom 11.5% reported unprotected extramarital sex. Unprotected extramarital sex and laboratory confirmed STIs decreased significantly in both intervention and control conditions over time. Results from unadjusted analysis show that the women in the C-POL intervention had a significantly lower rate of STI at 24 months (5.7%) compared to the control (8.3%; Fisher’s exact test, p=0.043). Figure 2 presents the percentage of any new STIs by condition over time for each gender.
Table 2.
Summary of behavioral and biological outcomes by condition
| Male n = 1775 | Female n = 2137 | Overall n = 3912 | ||||
|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | |
| Behavioral outcome | N=906 | N=869 | N=1073 | N=1064 | N=1979 | N=1933 |
| Any unprotected sex | ||||||
| Baseline | 103 (11.4) | 101 (11.6) | 25 (2.3) | 26 (2.4) | 128 (6.5) | 127 (6.6) |
| 12-month | 82 (10.8) | 58 (8.4) | 5 (0.5) | 12 (1.3) | 87 (5.0) | 70 (4.3) |
| 24-month | 59 (8.4) | 59 (8.8) | 4 (0.4) | 8 (0.9) | 63 (3.9) | 67 (4.2) |
| Biological outcome | N=905 | N=869 | N=1073 | N=1063 | N=1978 | N=1932 |
| Any (New) STIs | ||||||
| Baseline | 121 (13.4) | 134 (15.4) | 252 (23.5) | 258 (24.3) | 373 (18.9) | 392 (20.3) |
| 12-month | 29 (3.8) | 29 (4.2) | 81 (8.5) | 83 (9.0) | 110 (6.4) | 112 (6.9) |
| 24-month | 27 (3.8) | 22 (3.3) | 52 (5.7)* | 75 (8.3) | 79 (4.9) | 97 (6.2) |
N=Sample size at baseline.
Significantly fewer females in the intervention condition experienced any new STIs at 24-month follow-up than those in the control condition (Fisher’s exact test, p=0.043).
Figure 2.
Percentage of any new STIs by condition (intervention and control) and by gender across time (baseline, 12-month, and 24-month follow-ups).
Table 3 presents results from the Transition Model (model 1) testing the effect of previous STI status on follow-up STI incidence. Participants were less likely to acquire any new STIs if they were men (p<0.0001), older (p=0.0168) or had not previously experienced any STIs (p<0.0001). Participants in both study conditions who did not have a STI at baseline were unlikely to acquire a new STI at either follow-up assessment (<5% for intervention and control). There were significant intervention effects on incident STIs at 24 months among participants with previous STIs: 16.5% in the intervention markets compared to 29.5% in control. Results adjusting for age and gender indicated that the odds of acquiring any new STIs by the 24-month follow-up among those who experienced one or more STIs previously was 50% less in intervention markets compared to control markets (OR 0.47, 95% CI 0.25 to 0.90; p=0.0242). An intervention effect was also observed for women for all STIs (OR 0.44, 95% CI 0.20 to 0.95; p=0.0363) and for bacterial STIs (OR 0.46, 95% CI 0.23 to 0.90; p=0.0242).
Table 3.
Results of biological outcome and bacterial sexually transmitted infections (STIs) by previous STI status
| Previous STI status | ||||||
|---|---|---|---|---|---|---|
| No | Yes | |||||
| Intervention N (%) | Control N (%) | Established OR* (95% CI) Ref control |
Intervention N (%) | Control N (%) | Established OR* (95% CI) Ref control |
|
| Eligible* | ||||||
| Any new STIs | ||||||
| 12-month | 61 (4.3) | 65 (5.0) | 0.87 (0.54 to 1.41) | 49 (15.7) | 47 (14.5) | 1.08 (0.68 to 1.72) |
| 24-month | 61 (4.2) | 60 (4.2) | 0.99 (0.70 to 1.41) | 16 (16.5) | 31 (29.5) | 0.47 (0.25 to 0.90)* |
| Female† | ||||||
| Any new STIs | ||||||
| 12-month | 38 (5.1) | 42 (6.0) | 0.85 (0.47 to 1.55) | 43 (19.7) | 41 (18.4) | 1.08 (0.66 to 1.79) |
| 24-month | 38 (4.7) | 44 (5.5) | 0.84 (0.56 to 1.28) | 13 (18.8) | 27 (34.6) | 0.44 (0.20 to 0.95)* |
| Female† | ||||||
| Any bacterial STIs | ||||||
| 12-month | 40 (4.9) | 41 (5.4) | 0.90 (0.49 to 1.67) | 46 (29.3) | 47 (27.8) | 1.07 (0.67 to 1.71) |
| 24-month | 37 (4.3) | 34 (4.2) | 1.04 (0.64 to 1.67) | 22 (27.9) | 37 (45.7) | 0.46 (0.23 to 0.90)* |
Adjusted for age and gender; *p<0.05.
Adjusted for age; *p<0.05.
A similar trend was found when the same analytic method was applied to the behavioural outcome of self-reported unprotected extramarital sex. At baseline, participants in both conditions had similar rates of recent unprotected extramarital sex (6.5% intervention and 6.6% control shown in table 2), which was associated with STI at baseline (p=0.0052). Participants reporting behavioural risk at the 12-month follow-up reported lower rates of risk in the intervention (36%) compared to the control (42%) at the 24-month follow-up.
Finally, we examined the intervention effect on unprotected extramarital sex at the follow-up assessments for low versus high risk participants using model 2, which compares intervention and control participants stratified by their baseline STI status. Figure 3 presents the percentage reporting unprotected extramarital sex by study condition and baseline STI status. Among participants who had not experienced any STIs at baseline, the percentage having unprotected extramarital sex dropped in both conditions from baseline to 24-month follow-up from 5.5% to 3.8% in the intervention (OR 0.75, 95% CI 0.60 to 0.94; p<0.05) and from 6.5% to 3.9% in the control condition (OR 0.65; 95% CI 0.48 to 0.87; p<0.05) with no statistically significant differences in trends between conditions. However, among those who experienced one or more STIs at baseline, the percentage reporting unprotected extramarital sex dropped significantly from 10.7% to 4.5% (58% reduction) in the intervention markets (OR 0.38, 95% CI 0.19 to 0.77; p=0.0067) and non-significantly from 6.9% to 5.7% (17% reduction) in the control markets (OR 0.91, 95% CI 0.47 to 1.79). The control participants were more likely to engage in any unprotected extramarital sex than those in the intervention condition (OR 2.4, 95% CI 0.91 to 6.34; p=0.078) at 24-month follow-up.
Figure 3.
Percentage of unprotected sex by treatment and by baseline selected sexually transmitted infections.
DISCUSSION
The C-POL intervention had previously demonstrated efficacy when implemented with high-risk communities in the USA.3,4 A large, multi-site intervention trial in five countries was recently completed and the main outcome analyses did not show statistically significant intervention effects on STI or condom use with non-spousal partners.2 This paper presents results from the China site by demonstrating that the C-POL intervention did have significant impacts on STI incidence and behavioural risk among those most at risk for transmitting and acquiring STI—that is, those with diagnosed STI or unprotected sex with non-spousal partners. Investigators from the other C-POL sites may find similar results for their study samples using similar methods.
Previously, this team demonstrated that the C-POL intervention implemented in China shifted attitudes and reduced stigma around STI and HIV among the migrant market vendors in the intervention markets compared to control regardless of their risk level.11 The results suggest that the C-POL intervention succeeded in shifting social norms by diffusion of health messages through social networks. Community members in the intervention, but not the control communities, were significantly more likely to identify the intervention logo; to endorse routine clinic check-ups and condom use with non-marital partners; to talk to partners regarding sexual relationships; and to value the preservation of the health of one’s community.11 Although the results show a significant community shift in beliefs, attitudes and actions, public health officials are not impressed unless significant changes in biological outcomes are demonstrated.
One of the most important findings of this study is the specific populations with which community-level interventions are most efficacious—that is, among only the highest risk community members. Most efficacy trials select the highest risk members of a population to intervene with and include in study samples, which increases the likelihood of detecting statistically significant intervention effects. Since this efficacy trial examined a community-level intervention, at-risk communities were selected and not solely the highest risk individuals within those communities. The community level focus of the C-POL intervention required much larger sample sizes to enable examination of intervention impacts through diffusion among the entire community. Furthermore, intensive intervention of STI clinical and education services were provided to the control condition to meet ethical standards of research conduct and to isolate the effects of the C-POL intervention compared to clinical and educational services alone.
The relatively large samples in this study, and intensive intervention for controls, more closely resemble large scale effectiveness trials, which have had consistently disappointing results.12 Although the larger samples increase statistical power, statistically significant intervention effects are often masked by the relatively large proportion of low or moderate risk population members included.
In addition, many major randomised controlled trials for HIV prevention have demonstrated significant improvement in their control conditions resulting from repeated monitoring over time in addition to significant intervention for controls. Effect sizes in the control conditions can range from 15–30% reduction in risk.13–15 Thus, we must both reduce risk with lower risk participants and show reductions of at least 60% with the most risky participants–the initial 30% to match the changes in the control condition and at least another 30% change to be significantly greater in the intervention compared to the control. A similar challenge to demonstrating efficacy is presented by the spontaneous clearance of some STIs without treatment (ie, chlamydia).16–18 Spontaneous STI clearance is particularly problematic when there are long periods between study assessments such as in the C-POL trial in which there were 12-month intervals between assessments.
Generalisability
These findings should be generalisable to other populations in China and other countries, as well as other large scale, community-wide efficacy and effectiveness trials. Lessons learnt during the implementation of the intervention and assessment could be relevant to other randomised controlled trials and to evaluations of the C-POL intervention. The sampling and assessment procedures, monitoring of the quality of implementation and the outcomes were carefully documented and the findings were quite robust.
CONCLUSION
Community-level interventions are likely to be most relevant when all members of a population are in need of intervention. When a new disease is discovered (eg, H1N1 virus), knowledge and attitudes are the first targets of public health providers. National social marketing campaigns are examples of community-level interventions that have been highly successful in increasing knowledge and building positive attitudes.19 Yet, for behaviour change and biological outcomes, researchers are likely to be unable to demonstrate community level change except under conditions of a generalised epidemic.20 In this multisite trial of the C-POL intervention, intervention and control groups had similar STI incidence and behavioural risks over time when outcomes were analysed among all community members.2 Only when we examined differential intervention impacts for the highest risk participants compared to less risky participants did we find significant intervention effects.
These results suggest that inappropriate or unrealistic criteria are used when evaluating large scale community-level interventions and effectiveness trials. Yet, the solution is not to increase sample sizes for what are already very large study samples consisting of thousands of individuals. Evidence-based interventions typically succeed by reducing the riskiest behaviours among a small subgroup of people who repeatedly have STI and multiple sexual partners. Those with the most risk have the greatest opportunity for improvement. Except under conditions of generalised epidemics, the criteria for success of community-level interventions and large scale effectiveness trials should be broadened to include significant changes in attitudes, stigma and actions11 that are precursors to behavioural changes among the entire community. Significant reductions in STI incidence and high-risk behaviours can only be expected among the highest risk participants. Future studies must consider varying levels of risk within samples when evaluating intervention efficacy.
Acknowledgments
Funding This study was funded by the National Institute of Mental Health (NIMH) grant number U10MH61513: a five-country agreement conducted in China, India, Peru, Russia and Zimbabwe (trial registration: http://Clinicaltrials.gov NCT 00710060; registrant: Dr Li Li).
Footnotes
► An additional appendix is published online only. To view this file please visit the journal online (http://sti.bmj.com).
Competing interests None declared.
Ethics approval This study was conducted with the approval of the UCLA Internal Review Board; Institute Review Board of the National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention.
Contributors MJR-B was responsible for the programme design and funding, identified the analyses and wrote the final draft of the paper. ZW was responsible for the programme design in China, identified the analyses, provided oversight, training and quality control for supervision in the China site, and drafted and edited the paper. L-JL worked on the analytical models, analysed the data and wrote the initial results section. LL participated in and coordinated the US implementation of training, assessment, biological outcomes and quality control for the team, encouraging and shaping the intervention team’s skills on an ongoing basis. Additionally, she coordinated the data analyses with L-JL and the writing of the first draft of the paper. RD was responsible for the review of all STI data, reviews of the articles, on-site supervision of implementation, and reviewed and contributed to the writing of this paper. JG supervised the implementation in Fuzhou, China, of all aspects of the trial and reviewed and edited the paper. YY is the supervisor of all STI assessments in the central laboratory, supervised the implementation, training and analyses of the biological specimens, and edited the paper. DS assisted in preparing the paper for publication, editing the paper, and drafting the introduction and the discussion sections. The NIMH Collaborative HIV/STD Prevention Trial Group supported all aspects of a five-country study of HIV/STD prevention using the C-POL model. Each committee contributed to a different aspect of this study: the overall design, the assessment, the biological quality control, the intervention, the review of all preliminary data and the qualitative data collection.
Provenance and peer review Not commissioned; not externally peer reviewed.
REFERENCES
- 1.The NIMH Collaborative HIV/STD Prevention Trial Group. Methodological overview of a five-county community-level HIV/STD prevention trial. AIDS. 2007;21 Suppl 2:S3–S18. doi: 10.1097/01.aids.0000266453.18644.27. [DOI] [PubMed] [Google Scholar]
- 2.NIMH International HIV/STD Trial Group. Results of the NIMH Collaborative HIV/STD prevention trial of a community popular opinion leader intervention. J Acquir Immune Defic Syndr. 2010;54:204–214. doi: 10.1097/QAI.0b013e3181d61def. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelley JA, Murphy DA, Sikkema KJ, et al. Randomised, controlled, community-level HIV-prevention intervention for sexual-risk behaviour among homosexual men in US cities. Community HIV Prevention Research Collaborative. Lancet. 1997;350:1500–1505. doi: 10.1016/s0140-6736(97)07439-4. [DOI] [PubMed] [Google Scholar]
- 4.Sikkema KJ, Kelley JA, Winnett RA, et al. Outcomes of a randomized community-level HIV prevention intervention for women living in 18 low-income housing developments. Am J Pub Health. 2000;90:57–63. doi: 10.2105/ajph.90.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers EM. Diffusion of Innovations. 4th edn. New York, NY: Free Press; 1995. [Google Scholar]
- 6.Wu Z, Rotheram-Borus MJ, Detels R, et al. National Institute of Mental Health (NIMH) Collaborative HIV/STD Prevention Trial Group. Selecting at-risk populations for sexually transmitted disease/HIV intervention studies. AIDS. 2007;21 Suppl 8:S81–S87. doi: 10.1097/01.aids.0000304701.93002.00. [DOI] [PubMed] [Google Scholar]
- 7.Detels R, Wu Z, Rotheram MJ, et al. National Institute of Mental Health (NIMH) Collaborative HIV Prevention Trial Group. Sexually transmitted disease prevalence and characteristics of market vendors in eastern China. Sex Transm Dis. 2003;30:803–808. doi: 10.1097/01.OLQ.0000086607.82667.CF. [DOI] [PubMed] [Google Scholar]
- 8.NIMH Collaborative HIV/STD Prevention Trial Group. Selection of populations represented in the NIMH Collaborative HIV/STD Prevention Trial. AIDS. 2007;21 Suppl 2:S19–S28. doi: 10.1097/01.aids.0000266454.26268.90. [DOI] [PubMed] [Google Scholar]
- 9.NIMH Collaborative HIV/STD Prevention Trial Group. The community popular opinion leader HIV prevention programme: conceptual basis and intervention procedures. AIDS. 2007;21 Suppl 2:S59–S68. doi: 10.1097/01.aids.0000266458.49138.fa. [DOI] [PubMed] [Google Scholar]
- 10.Zeger SL, Liang KY, Self SG. The analysis of binary longitudinal data with time-independent covariates. Bionetrika. 1965;72:31–38. [Google Scholar]
- 11.Li L, Liang LJ, Lin CQ, et al. HIV prevention intervention to reduce HIV-related stigma: evidence from China. AIDS. 2010;24:115–122. doi: 10.1097/QAD.0b013e3283313e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rotheram MJ, Swendeman D, Chovnick G. The past, present, and future of HIV prevention: integrating behavioral, biomedical, and structural intervention strategies for the next generation of HIV prevention. Annu Rev Clin Psychol. 2009;5:143–167. doi: 10.1146/annurev.clinpsy.032408.153530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lightfoot M, Rotheram-Borus MJ, Comulada S, et al. Self-monitoring of behavior as a risk reduction strategy for persons living with HIV. AIDS Care. 2007;19:757–763. doi: 10.1080/09540120600971117. [DOI] [PubMed] [Google Scholar]
- 14.Lighfoot M, Comulada WS, Stover G. Computerized preventive intervention for adolescents: indications of efficacy. Am J Public Health. 2007;97:1027–1030. doi: 10.2105/AJPH.2005.072652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamb ML, Fishbein M, Douglas JM, Jr, et al. Efficacy of risk-reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: a randomized controlled trial. Project RESPECT Study Group. JAMA. 1998;280:1161–1167. doi: 10.1001/jama.280.13.1161. [DOI] [PubMed] [Google Scholar]
- 16.Brunham RC, Rey-Ladino J. Immunology of chlamydia infection: implication for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5:149–161. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 17.Golden MR, Schillinger JA, Markowitz L, et al. Duration of untreated genital infections with Chlamydia trachomatis; a review of the literature. Sex Transm Dis. 2000;27:329–337. doi: 10.1097/00007435-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Parks KS, Dixon PB, Richey CM, et al. Spontaneous clearance of Chlamydia trachomatis infection in untreated patients. Sex Transm Dis. 1997;24:229–235. doi: 10.1097/00007435-199704000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Frech M. Evaluation der nationalen STOP AIDS-Kampagne 2005. mrc. Zug, Switzerland, 2006. [accessed 12 Jun 2010]; http://www.bag.admin.ch/evaluation/01759/02062/02241/index.html?lang=en#. [Google Scholar]
- 20.Patterson T. Brief interventions for reducing HIV. Paper presented at the University of California, Center for HIV Identification, Prevention, Treatment, and Services; June 2008; Los Angeles, California, USA. [Google Scholar]