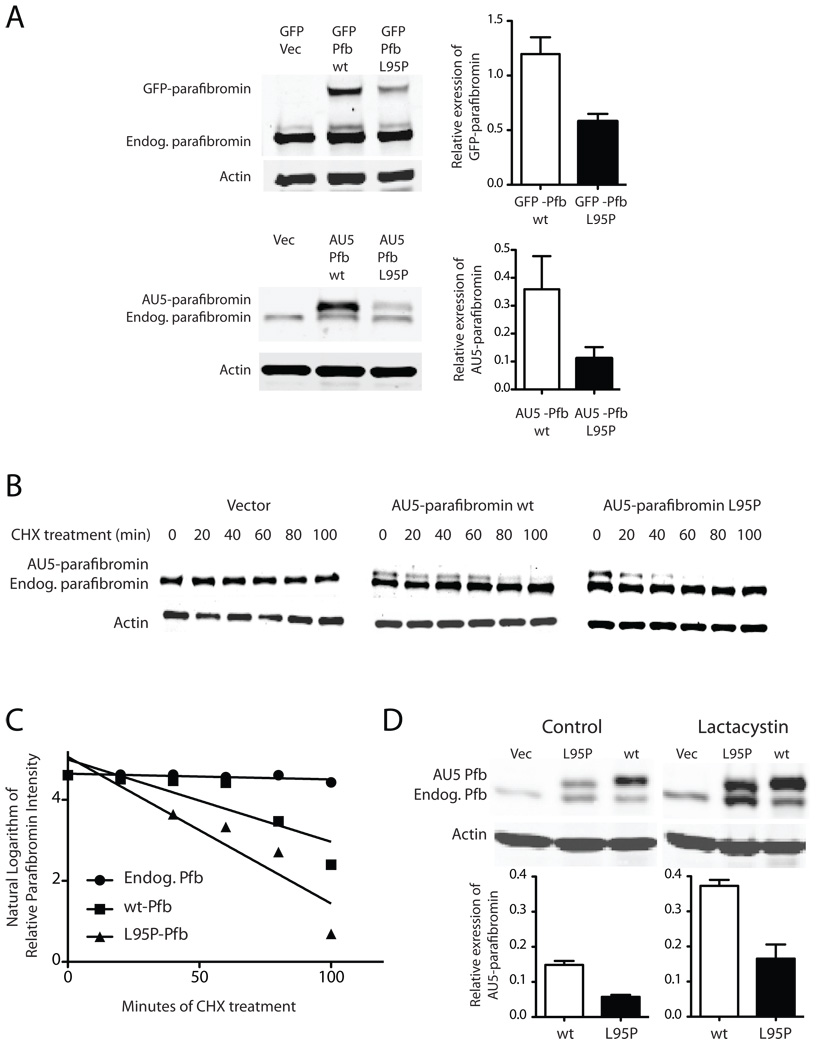
Figure 2. Diminished expression level of the L95P parafibromin mutant not due to accelerated proteasomal degradation.
A (Left) Immunoblots of GFP control or GFP fusions with wild-type (wt) or L95P mutant parafibromin (Pfb) (upper panels) and vector control (Vec), or AU5 epitope-tagged wild-type or L95P mutant parafibromin (lower panels) and endogenous parafibromin in cultured Hela cells after 24 hours of transfection. (Right) Histograms quantifying the relative expression level of wild type and L95P mutant parafibromin constructs compared to β-actin in the same sample. B. Time course of protein expression of endogenous parafibromin, AU5-parafibromin and AU5 L95P-parafibromin following cycloheximide treatment to block de novo protein synthesis. Cultured HeLa cells were transfected with the indicated constructs, then 6 hours following transfection cycloheximide was added to the culture medium and cells were collected at 0, 20, 40, 60, 80 and 100 min. Cell lysates were analyzed by immunoblotting at the indicated time points using anti-parafibromin antibody (upper) or anti-actin (lower) antibodies. The expression of β-actin is shown as a loading control. C. Linear regression analysis of the relative expression of transfected and endogenous parafibromin from (B) as described in Experimental Procedures. D. Immunoblots (above) and histograms (below) showing the expression, relative to β-actin, of wild type and L95P mutant AU5-tagged parafibromin without and with treatment with the proteasome inhibitor lactacystin for two hours.