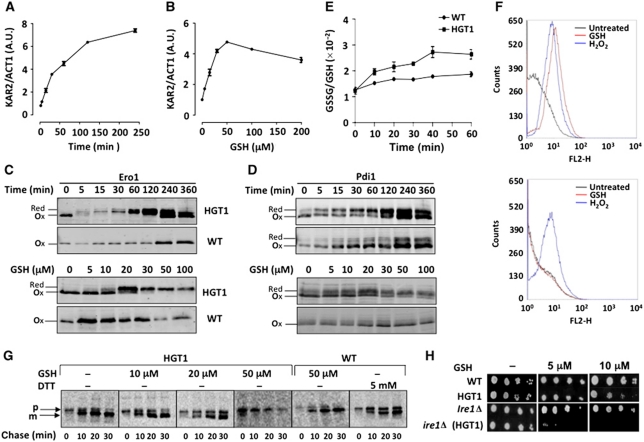
Figure 4.
GSH toxic levels block secretion by ER reductive load. KAR2 expression monitored by RT–PCR as in Figure 3A in (A) exponentially growing HGT1 cells at the indicated time after adding 100 μM GSH or (B) 30 min after adding the indicated amount of GSH (μM). FET3 expression is given as FET3/ACT1 signal ratio. Values are the mean of triplicate samples of the same experiment±s.d. (C) HGT1 or WT cells expressing Myc-tagged Ero1 exposed to 50 μM GSH for the indicated time (upper panel) or for 30 min with the indicated amount of GSH (lower panel), and processed for redox western with anti-Myc antibodies. (D) As in (C), except that the western blot revealed with Pdi1-specific antibodies. (E) Fifteen minutes after adding GSH (50 μM) to HGT1 or WT cell cultures, 25 mM DTT was added. After 15 min, cells were collected, washed and inoculated in fresh SD medium at the same OD. Samples were collected for GSH and GSSG measurements. Data are the mean of three independent experiments±s.d. (F) HGT1 (upper panel) or ire1Δ cells carrying HGT1 (lower panel) incubated for 1 h into PBS containing dihydrorhodamine 123 and either GSH (100 μM) or H2O2 (400 μM), or left untreated, and analysed by flow cytometry. Data are typical of three independent experiments. (G) HGT1 and WT cells incubated during 30 min with the indicated amount of GSH, pulsed with 35S-methionine for 7 min and chased. Cpy was immunoprecipitated from cells collected at 0, 10, 20 and 30 min after chase initiation, separated by SDS–PAGE and revealed by phosphor technology. Arrows indicate Cpy immature (p) and mature vacuolar (m) forms. In last panel, WT cells treated with 5 mM DTT for 15 min, and processed, as above. (H) Exponentially growing WT or ire1Δ cells carrying pTEF-416-HGT1 or empty vector serially diluted and spotted onto SD plates containing the indicated amount of GSH.