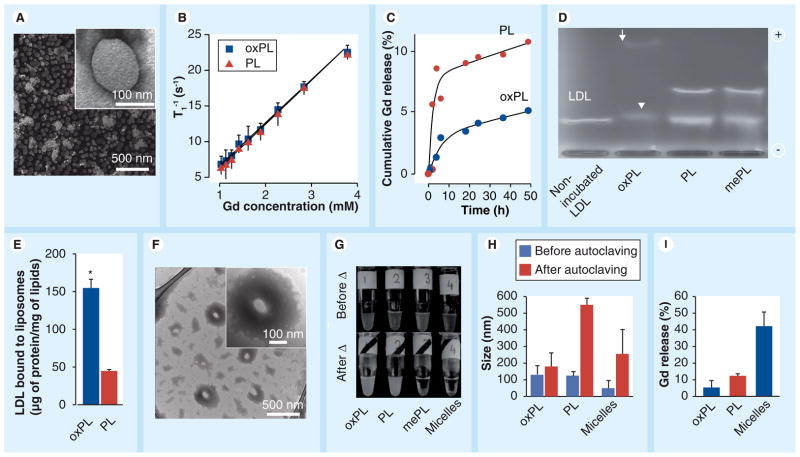
Figure 2. Characterization of vesicles containing 9-CCN (oxPL) and control (PL).
(A) Electron micrographs of oxPL show round unilamellar vesicles approximately 100 nm in diameter (inset). (B) relaxivity of oxPL and PL containing different Gd concentrations at T1 1.5 T. (C) In vitro Gd release in human plasma as measured by inductively coupled plasma mass spectrometry. (D) Agarose electrophoresis of human LDL incubated with vesicle formulations. The arrow indicates a highly mobile electronegative band while the arrow head indicates slightly higher electrophoretic mobility, but with lower densitometric concentration of LDL. (E) LDL was incubated with oxPL or PL followed by ultracentrifugation and quantification of LDL protein. (F) Vesicles were incubated with LDL followed by transmission electron microscopy. oxPL tends to accumulate LDL lipids on its surface resulting in lipid ‘cloud’ formation around the core (inset). (G) Photographs of vesicle preparations and miceller solutions before and after autoclaving. Moderate aggregation was observed for oxPL while complete water–lipid phase separation was seen for micelles and mePL. (H) Particle size by dynamic light scattering before and after autoclaving. (I) Following autoclaving all formulations were subjected to exhaustive dialysis and Gd release was determined as a ratio of the final Gd concentrations to the concentrations obtained before experiment. *p < 0.01
Gd: Gadolinium; LDL: Low-density lipoprotein; mePL: (3b)-cholest-5-en-3-yl methyl azelaate phospholipid vesicles; oxPL: Cholesteryl-9-carboxynonanoate phospholipid vesicles; PL: Phospholipid.