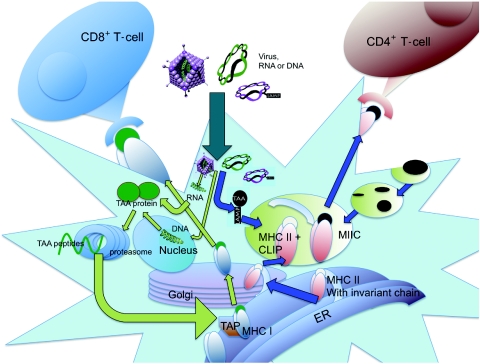
Figure 1.
Pathways of antigen processing and presentation. Endogenous proteins are degraded in the cytoplasm by the proteasome. Cleaved peptides are ushered into the endoplasmic reticulum by TAP (transporter associated with antigen processing), where they are loaded onto preformed MHC I/β2m complexes. Stable MHC I:peptide binding allows the complexes to traffic via the Golgi to the cell surface for antigen presentation to CD8+ T-cells. MHC II molecules are formed in the endoplasmic reticulum (ER) and traffic through the Golgi. The invariant chain is used to prevent binding of “self” peptides and to stabilize the MHC II complex. Upon entry into the MHC II compartments (MIIC), the invariant chain is degraded, leaving a small, class II-associated peptide (CLIP). Within the MIIC, the CLIP is replaced with peptides resulting from degradation of endocytosed pathogens. For ectopic expression, genes can be introduced by virus infection or RNA/DNA transfection. Unless otherwise modified, proteins expressed by either strategy are typically processed by the proteasome and presented on MHC I molecules. However, proteins can be also targeted to the MHC II pathway by tagging with sorting signals, including lysosome-associated membrane protein-1 (LAMP-1). TAA, tumor-associated antigen.