Figure 4.
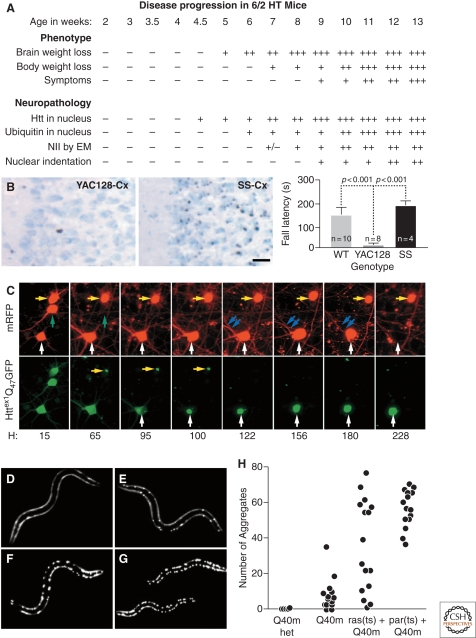
HD pathogenesis unfolds as a complex temporal dynamic, instigated by polyQ-expanded huntingtin but shaped by a network of cellular adaptive responses. (A) In the R6/2 mouse model of HD, IB formation precedes and correlates to behavioral deficits. Adapted with permission from (Davies et al. 1997). However, the Shortstop mouse model of HD (B) has more IBs (left, micrographs) but fewer behavioral deficits (right, graph) than the YAC128 mouse model of HD. Adapted with permission from (Slow et al. 2005). (C) The paradox could be explained if IB formation behaves as a coping response to more diffuse forms of mutant huntingtin. Here striatal neurons were transfected with mutant huntingtin tagged with GFP and followed longitudinally. Levels of diffuse mutant huntingtin decrease to nearly baseline levels after IB formation and, at the same, begin to show a lower risk of death (i.e., better survival). Adapted with permission from Arrasate et al. (2004). (D–H) Progressive disruption of cellular folding capacity by misfolded proteins. Fluorescent images of representative L2 larvae at the permissive temperature of heterozygous (D) or homozygous (E) Q40 m, ras (ts)+Q40 m (F), and paramyosin (ts)+Q40 m (G) strains. (H) Number of visible aggregates in L2 larvae expressing indicated proteins. ras (ts)+Q40 m in (F) and (G) denotes the fluorescent progeny of an F2 ras (ts) animal expressing Q40 m; these progeny could be either homozygous or heterozygous for Q40 m. Adapted with permission from Gidalevitz et al. (2006).