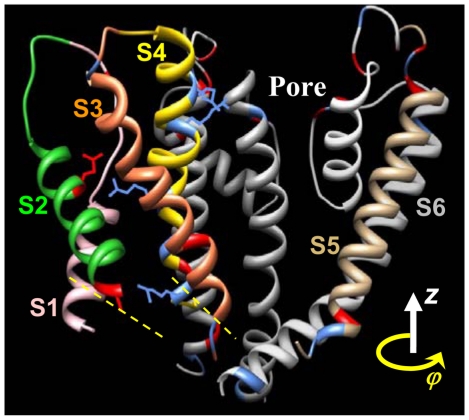
Figure 3. 3D structure of Kv7.1 (one subunit) and its transmembrane helices in the open conformation.
The structure was computed based on homology with Kv1.2 using its known crystal structure [13]. Motion of the S4–S3 complex is assumed to be the major conformational change during channel opening and closing (gating). The loop connecting S2 to S3 and the linker connecting S4 to S5 are not shown (dashed lines). Dark gray helices are S5 and S6 of the neighboring subunit. Red segments are negatively charged residues and blue segments are positively charged residues.