Figure 4. Modification of Stat3 by C48.
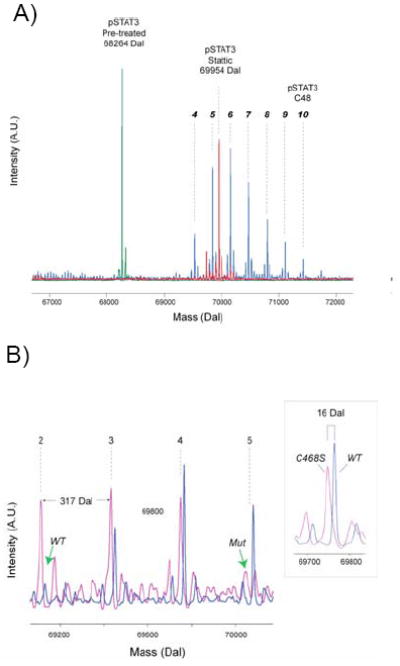
A) Mass spectra of phosphorylated Stat3 treated with C48 and Stattic. Quadrupole time of flight mass spectra of phosphorylated Stat3 before treatment (green trace) and after treatment with C48 (blue trace) and Stattic (red trace). Multiple modifications are observed for Stat3. Based on the expected mass of the C48 fragment, more than 6 modifications were observed corresponding to 4 to 10 C48 adducts. Phosphorylated Stat3 treated with Stattic under the same conditions produce a predominant peak at 69954, which corresponds to the modification of precisely 8 residues.
B) Mutation of Cys468 reduces the number of C48 modifications. Wildtype Stat3 (blue trace) and C468S Stat3 mutant (pink trace), both unphosphorylated, were treated with C48 for one minute at 37 °C and analyzed by mass spectrometry. For wildtype Stat3, the mass spectrum indicates 3 adducts comprising 3, 4 and 5 modifications. For the C468S mutant, the mass spectrum also indicates 3 adducts, but consists of 2, 3 and 4 modifications. The green arrows indicate absence of modifications on wildtype Stat3 (wt) and the mutant Stat3 (mut). The inset shows the mass difference between the wildtype and mutant is precisely 16 daltons, as expected.
