Figure 1. Schematic summary of the role of TRP channels in cells of the cardiovascular system.
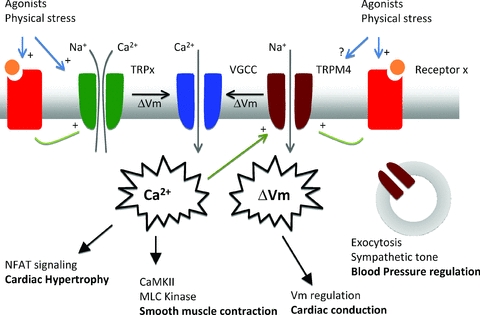
Roughly TRP channels can be subdivided in two groups: Ca2+ permeable and Ca2+ impermeable cation channels. Both will have an effect on intracellular Ca2+ dynamics, either directly by providing a Ca2+ influx pathway, or indirectly through membrane depolarisation, activation of voltage-gated Ca2+ channels and/or influencing the driving force for Ca2+ entry. TRP channels might be activated directly by agonists, or indirectly through G-protein coupled receptors. TRPC channels are Ca2+ permeable channels activated by G-protein coupled receptors (GPCR), through Gq and phospholipase C. The resulting Ca2+ influx apparently regulates specifically NFAT signalling and development of cardiac hypertrophy, without changing the Ca2+ transient during normal heart cycle. Ca2+ influx either through TRP channels or voltage-gated Ca2+ channel activation after TRP channel mediated depolarization is linked with myosin light chain kinase phosphorylation and smooth muscle contraction during the development of myogenic tone. A mechanically activated GPCR is linked with activation of TRPC6 and possibly TRPM4. Membrane potential regulation by TRPM4 in cardicac Purkinje cells might be important for proper cardiac conduction of the action potential through the heart. Through an unknown mechanism, TRPM4 also plays a role in the exocytosis of adrenaline from chromaffin cells, regulating in this way the sympathetic control of blood pressure. For more details and references, see the text.
